J Korean Soc Transplant.
2016 Sep;30(3):138-142. 10.4285/jkstn.2016.30.3.138.
Falsely Elevated Tacrolimus Concentrations Using Chemiluminescence Microparticle Immunoassay in Kidney Transplant Patient
- Affiliations
-
- 1Department of Laboratory Medicine, Kosin University College of Medicine, Busan, Korea. lukerubicon@gmail.com
- 2Department of Laboratory Medicine, Inje University College of Medicine, Busan, Korea.
- 3Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea.
- KMID: 2354638
- DOI: http://doi.org/10.4285/jkstn.2016.30.3.138
Abstract
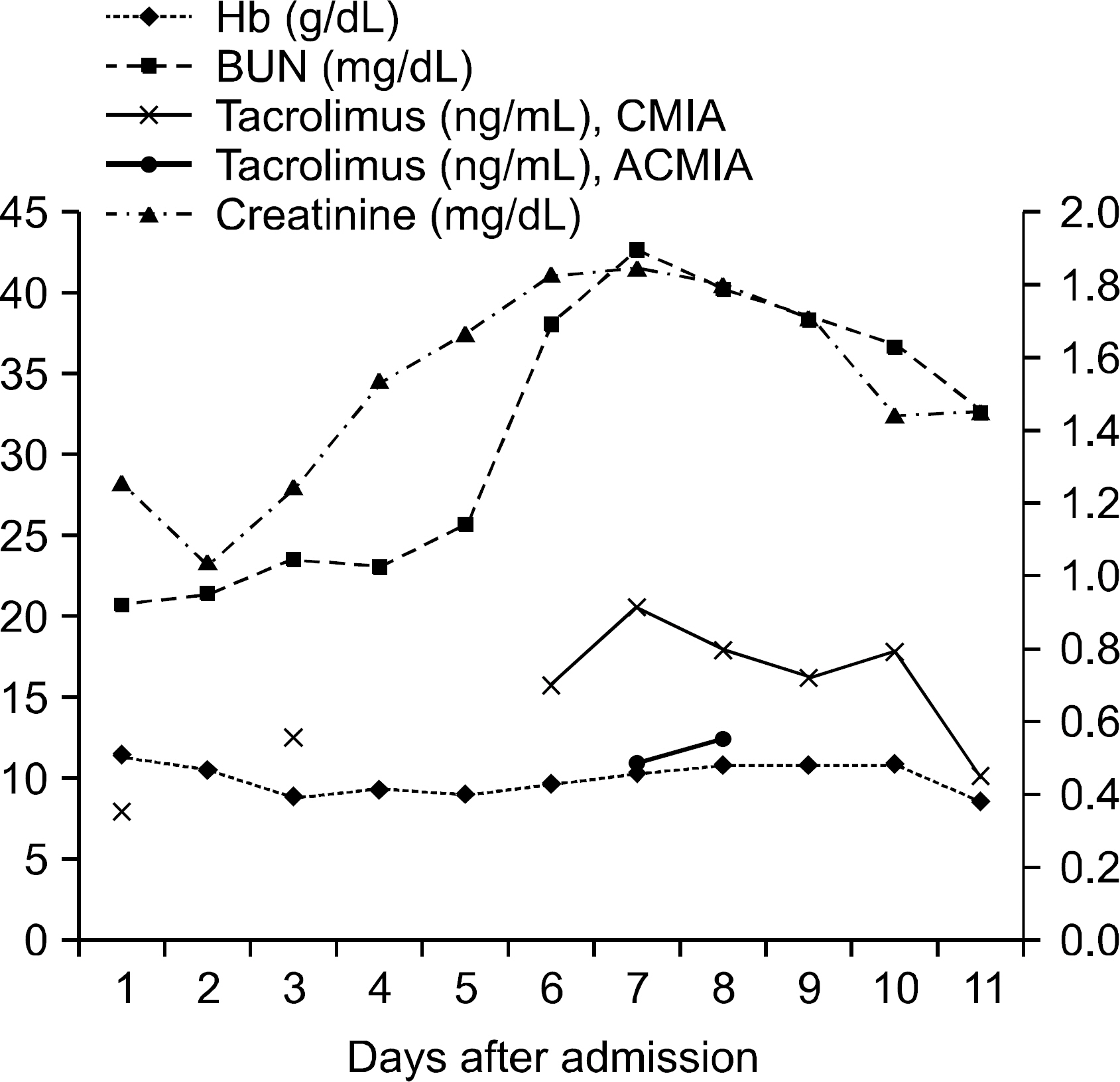
- Tacrolimus is one of the effective immunosuppressive drugs used after an organ transplant procedure. However, due to its narrow therapeutic range, its usefulness in preventing transplant rejection and minimizing nephrotoxicity is dependent on the monitoring of whole blood trough levels of tacrolimus. A 49-year-old kidney transplant recipient presenting with cough and general weakness was admitted to the hospital. Due to the patient's deeply compromised clinical condition, an immunosuppressive therapy was discontinued. Tacrolimus concentrations in the patient's whole blood samples were measured, using an automated chemiluminescent microparticle immunoassay (CMIA) instrument. Interference was suspected because tacrolimus concentrations after the discontinuation of tacrolimus dose were 20.9 and 18.2 ng/mL at day 2 and 3, respectively. Tacrolimus concentrations were 11.1 and 12.6 ng/mL, respectively, when re-tested using an antibody-conjugated magnetic immunoassay (ACMIA). We evaluated the relationship between the CMIA and ACMIA results, and calculated the expected values from the regression equation. Residuals were -8.4 and -4 ng/mL, respectively. There have been several cases with false detection of elevated tacrolimus concentrations using ACMIA; however, such falsely detected elevations using CMIA have rarely been reported. When unexpectedly high concentrations of tacrolimus are detected by CMIA in transplant patients, an immediate re-test using another technique might be necessary to rule out falsely elevated results.
Keyword
MeSH Terms
Figure
Reference
-
1). Knoll G. Trends in kidney transplantation over the past decade. Drugs. 2008. 68(Suppl 1):3–10.
Article2). Pascual M., Theruvath T., Kawai T., Tolkoff-Rubin N., Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002. 346:580–90.
Article3). O'Grady JG., Burroughs A., Hardy P., Elbourne D., Truesdale A. UK and Republic of Ireland Liver Transplant Study Group. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet. 2002. 360:1119–25.4). United States Organ Transplantation. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2011 annual data report [Internet]. Rockville: United States Organ Transplantation;2012. [cited 2016 Aug 9]. Available from:. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/00_intro_12.pdf.5). Schiff J., Cole E., Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007. 2:374–84.
Article6). Undre NA., van Hooff J., Christiaans M., Vanrenterghem Y., Donck J., Heeman U, et al. Low systemic exposure to tacrolimus correlates with acute rejection. Transplant Proc. 1999. 31:296–8.
Article7). Henry JB., McPherson RA, et al. Henry's clinical diagnosis and management by laboratory methods. 22nd ed.Philadelphia, PA: Elsevier/Saunders;2011.8). Tate J., Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004. 25:105–20.9). Li ZY., Yan CL., Yan R., Feng ZR. Analytical performance of the Abbott Architect i2000 tacrolimus assay in Chinese patients after renal transplantation. Transplant Proc. 2010. 42:4534–7.
Article10). Munshi SU., Anwar A., Tabassum S. False positive human immunodeficiency virus antibody test in chronic hepatitis B patient. Indian J Med Microbiol. 2014. 32:344–5.
Article11). Wallemacq PE., Verbeeck RK. Comparative clinical pharmacokinetics of tacrolimus in paediatric and adult patients. Clin Pharmacokinet. 2001. 40:283–95.
Article12). Napoli KL. Is microparticle enzyme-linked immunoassay (MEIA) reliable for use in tacrolimus TDM? Comparison of MEIA to liquid chromatography with mass spectrometric detection using longitudinal trough samples from transplant recipients. Ther Drug Monit. 2006. 28:491–504.
Article13). Seger C. Usage and limitations of liquid chromatography-tandem mass spectrometry (LC-MS/MS) in clinical routine laboratories. Wien Med Wochenschr. 2012. 162:499–504.
Article14). Vogeser M., Seger C. Pitfalls associated with the use of liquid chromatography-tandem mass spectrometry in the clinical laboratory. Clin Chem. 2010. 56:1234–44.
Article15). Koster RA., Dijkers EC., Uges DR. Robust, high-throughput LC-MS/MS method for therapeutic drug monitoring of cyclosporine, tacrolimus, everolimus, and sirolimus in whole blood. Ther Drug Monit. 2009. 31:116–25.
Article16). Holt DW., Armstrong VW., Griesmacher A., Morris RG., Napoli KL., Shaw LM, et al. International Federation of Clinical Chemistry/International Association of Therapeutic Drug Monitoring and Clinical Toxicology working group on immunosuppressive drug monitoring. Ther Drug Monit. 2002. 24:59–67.
Article17). Wallemacq P., Armstrong VW., Brunet M., Haufroid V., Holt DW., Johnston A, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009. 31:139–52.
Article18). De BK., Jimenez E., De S., Sawyer JC., McMillin GA. Analytical performance characteristics of the Abbott Architect i2000 Tacrolimus assay: comparisons with liquid chromatography-tandem mass spectrometry (LC-MS/MS) and Abbott IMx methods. Clin Chim Acta. 2009. 410:25–30.
Article19). Amann S., Parker TS., Levine DM. Evaluation of 2 immunoassays for monitoring low blood levels of tacrolimus. Ther Drug Monit. 2009. 31:273–6.
Article20). Wallemacq P., Goffinet JS., O'Morchoe S., Rosiere T., Maine GT., Labalette M, et al. Multi-site analytical evaluation of the Abbott ARCHITECT tacrolimus assay. Ther Drug Monit. 2009. 31:198–204.
Article21). Bazin C., Guinedor A., Barau C., Gozalo C., Grimbert P., Duvoux C, et al. Evaluation of the Architect tacrolimus assay in kidney, liver, and heart transplant recipients. J Pharm Biomed Anal. 2010. 53:997–1002.
Article22). Chung JW., An D., Song J., Chung HJ., Park HI., Lee W, et al. Performance evaluation of affinity column mediated im-munometric assay for tacrolimus. Korean J Lab Med. 2009. 29:415–22. (정재우, 안동희, 송정한, 정희정, 박해일, 이우 창, 등. Affinity Column Mediated Immunometric Assay를 이용한 혈중 Tacrolimus 농도 검사법의 평가. 대한진단검사의학 회지 2009;29: 415-22.).
Article23). D'Alessandro M., Mariani P., Mennini G., Severi D., Berloco P., Bachetoni A. Falsely elevated tacrolimus concentrations measured using the ACMIA method due to circulating endogenous antibodies in a kidney transplant recipient. Clin Chim Acta. 2011. 412:245–8.24). Knorr JP., Grewal KS., Balasubramanian M., Young N., Zaki R., Khanmoradi K, et al. Falsely elevated tacrolimus levels caused by immunoassay interference secondary to beta-galactosidase antibodies in an infected liver transplant recipient. Pharmacotherapy. 2010. 30:954.25). Altinier S., Varagnolo M., Zaninotto M., Boccagni P., Plebani M. Heterophilic antibody interference in a non-endogenous molecule assay: an apparent elevation in the tacrolimus concentration. Clin Chim Acta. 2009. 402:193–5.
Article26). Rostaing L., Cointault O., Marquet P., Josse AG., Lavit M., Saint-Marcoux F, et al. Falsely elevated whole-blood tacrolimus concentrations in a kidney-transplant patient: potential hazards. Transpl Int. 2010. 23:227–30.
Article27). Cha KH., Lee JJ., Kim HN., Chae H., Kim Y. False increase in whole blood tacrolimus levels due to interference in an antibody-conjugated magnetic immunoassay method. J Lab Med Qual Assur. 2015. 37:148–52. (차경호, 이정중, 김한 나, 채효진, 김용구. Antibody Conjugated Magnetic Immun-oassay법을 이용한 Tacrolimus 농도분석에서 간섭으로 인한 위 증가 2예. 임상검사와 정도관리 2015;37: 148-52.).
Article28). Murthy JN., Davis DL., Yatscoff RW., Soldin SJ. Tacrolimus metabolite cross-reactivity in different tacrolimus assays. Clin Biochem. 1998. 31:613–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- False Increase in Whole Blood Tacrolimus Levels due to Interference in an Antibody-Conjugated Magnetic Immunoassay Method
- Risk of graft loss on once-daily versus twice-daily tacrolimus in kidney transplant patients: a meta-analysis
- Effect of tacrolimus XL on variance coefficients in comparison with twice daily tacrolimus, and relationship with serum creatinine concentrations in kidney transplant recipients
- The comparison of coefficient of variation among once-daily and twice-daily tacrolimus in kidney transplant patients: a meta-analysis
- Performance Evaluation of the ARCHITECT i2000 for the Determination of Whole Blood Cyclosporin A and Tacrolimus