J Pathol Transl Med.
2016 Sep;50(5):345-354. 10.4132/jptm.2016.06.14.
Differential Immunohistochemical Profiles for Distinguishing Prostate Carcinoma and Urothelial Carcinoma
- Affiliations
-
- 1Department of Hospital Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. mdyjchoi@catholic.ac.kr
- 2Department of Urology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2353596
- DOI: http://doi.org/10.4132/jptm.2016.06.14
Abstract
- BACKGROUND
The pathologic distinction between high-grade prostate adenocarcinoma (PAC) involving the urinary bladder and high-grade urothelial carcinoma (UC) infiltrating the prostate can be difficult. However, making this distinction is clinically important because of the different treatment modalities for these two entities.
METHODS
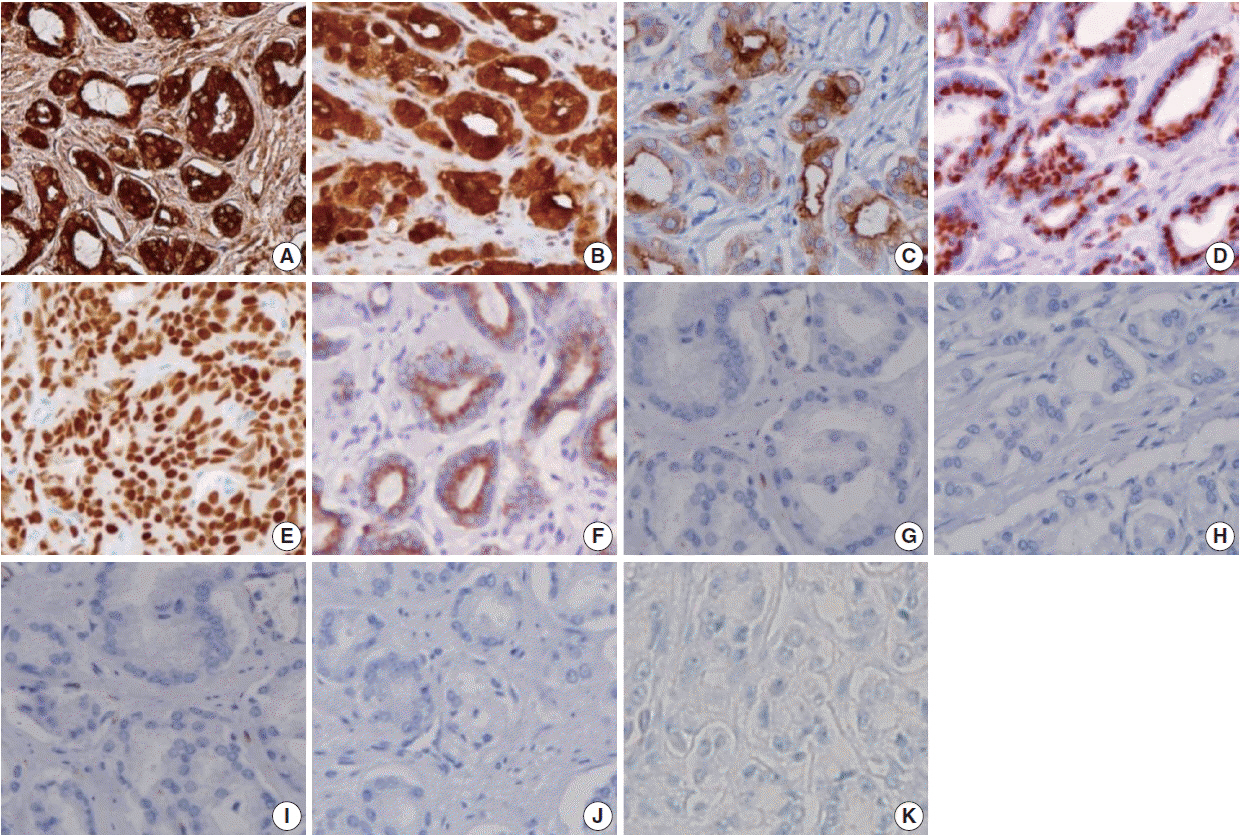
A total of 249 patient cases (PAC, 111 cases; UC, 138 cases) collected between June 1995 and July 2009 at Seoul St. Mary's Hospital were studied. An immunohistochemical evaluation of prostatic markers (prostate-specific antigen [PSA], prostate-specific membrane antigen [PSMA], prostate acid phosphatase [PAP], P501s, NKX3.1, and α-methylacyl coenzyme A racemase [AMACR]) and urothelial markers (CK34βE12, p63, thrombomodulin, S100P, and GATA binding protein 3 [GATA3]) was performed using tissue microarrays from each tumor.
RESULTS
The sensitivities of prostatic markers in PAC were 100% for PSA, 83.8% for PSMA, 91.9% for PAP, 93.7% for P501s, 88.3% for NKX 3.1, and 66.7% for AMACR. However, the urothelial markers CK34βE12, p63, thrombomodulin, S100P, and GATA3 were also positive in 1.8%, 0%, 0%, 3.6%, and 0% of PAC, respectively. The sensitivities of urothelial markers in UC were 75.4% for CK34βE12, 73.9% for p63, 45.7% for thrombomodulin, 22.5% for S100P, and 84.8% for GATA3. Conversely, the prostatic markers PSA, PSMA, PAP, P501s, NKX3.1, and AMACR were also positive in 9.4%, 0.7%, 18.8%, 0.7%, 0%, and 8.7% of UCs, respectively.
CONCLUSIONS
Prostatic and urothelial markers, including PSA, NKX3.1, p63, thrombomodulin, and GATA3 are very useful for differentiating PAC from UC. The optimal combination of prostatic and urothelial markers could improve the ability to differentiate PAC from UC pathologically.
Keyword
MeSH Terms
Figure
Reference
-
1. Bates AW, Baithun SI. Secondary neoplasms of the bladder are histological mimics of nontransitional cell primary tumours: clinicopathological and histological features of 282 cases. Histopathology. 2000; 36:32–40.
Article2. Dabbs DJ. Diagnostic imunohistochemistry. 3rd ed. Philadelphia: Saunders-Elsevier;2010. p. 621–5.3. Chuang AY, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. Am J Surg Pathol. 2007; 31:1246–55.
Article4. Varma M, Jasani B. Diagnostic utility of immunohistochemistry in morphologically difficult prostate cancer: review of current literature. Histopathology. 2005; 47:1–16.
Article5. Mhawech P, Uchida T, Pelte MF. Immunohistochemical profile of high-grade urothelial bladder carcinoma and prostate adenocarcinoma. Hum Pathol. 2002; 33:1136–40.
Article6. Kunju LP, Mehra R, Snyder M, Shah RB. Prostate-specific antigen, high-molecular-weight cytokeratin (clone 34betaE12), and/or p63: an optimal immunohistochemical panel to distinguish poorly differentiated prostate adenocarcinoma from urothelial carcinoma. Am J Clin Pathol. 2006; 125:675–81.7. Chibber PJ, McIntyre MA, Hindmarsh JR, Hargreave TB, Newsam JE, Chisholm GD. Transitional cell carcinoma involving the prostate. Br J Urol. 1981; 53:605–9.
Article8. Kamoshida S, Tsutsumi Y. Extraprostatic localization of prostatic acid phosphatase and prostate-specific antigen: distribution in cloacogenic glandular epithelium and sex-dependent expression in human anal gland. Hum Pathol. 1990; 21:1108–11.9. Goldstein NS. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am J Clin Pathol. 2002; 117:471–7.
Article10. Genega EM, Hutchinson B, Reuter VE, Gaudin PB. Immunophenotype of high-grade prostatic adenocarcinoma and urothelial carcinoma. Mod Pathol. 2000; 13:1186–91.
Article11. Marchal C, Redondo M, Padilla M, et al. Expression of prostate specific membrane antigen (PSMA) in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. Histol Histopathol. 2004; 19:715–8.12. Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998; 82:2256–61.13. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997; 3:81–5.14. Chang SS, Reuter VE, Heston WD, Gaudin PB. Comparison of anti-prostate-specific membrane antigen antibodies and other immunomarkers in metastatic prostate carcinoma. Urology. 2001; 57:1179–83.
Article15. Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998; 52:637–40.
Article16. Lane Z, Hansel DE, Epstein JI. Immunohistochemical expression of prostatic antigens in adenocarcinoma and villous adenoma of the urinary bladder. Am J Surg Pathol. 2008; 32:1322–6.
Article17. Bassily NH, Vallorosi CJ, Akdas G, Montie JE, Rubin MA. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000; 113:383–8.
Article18. Xu J, Kalos M, Stolk JA, et al. Identification and characterization of prostein, a novel prostate-specific protein. Cancer Res. 2001; 61:1563–8.19. Kalos M, Askaa J, Hylander BL, et al. Prostein expression is highly restricted to normal and malignant prostate tissues. Prostate. 2004; 60:246–56.
Article20. He WW, Sciavolino PJ, Wing J, et al. A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics. 1997; 43:69–77.
Article21. Bowen C, Bubendorf L, Voeller HJ, et al. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res. 2000; 60:6111–5.22. Gelmann EP, Bowen C, Bubendorf L. Expression of NKX3.1 in normal and malignant tissues. Prostate. 2003; 55:111–7.
Article23. Aslan G, Irer B, Tuna B, Yorukoglu K, Saatcioglu F, Celebi I. Analysis of NKX3.1 expression in prostate cancer tissues and correlation with clinicopathologic features. Pathol Res Pract. 2006; 202:93–8.
Article24. Jiang Z, Li C, Fischer A, Dresser K, Woda BA. Using an AMACR (P504S)/34betaE12/p63 cocktail for the detection of small focal prostate carcinoma in needle biopsy specimens. Am J Clin Pathol. 2005; 123:231–6.25. Parker DC, Folpe AL, Bell J, et al. Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. Am J Surg Pathol. 2003; 27:1–10.
Article26. Parwani AV, Kronz JD, Genega EM, Gaudin P, Chang S, Epstein JI. Prostate carcinoma with squamous differentiation: an analysis of 33 cases. Am J Surg Pathol. 2004; 28:651–7.27. Comperat E, Camparo P, Haus R, et al. Immunohistochemical expression of p63, p53 and MIB-1 in urinary bladder carcinoma: a tissue microarray study of 158 cases. Virchows Arch. 2006; 448:319–24.
Article28. Higgins JP, Kaygusuz G, Wang L, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007; 31:673–80.
Article29. Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012; 36:1472–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prostate specific antigen in poorly differentiation tumors of bladder neck
- Seminal Vesicle Involvement by Urothelial Carcinoma in Situ of the Bladder with Mucosal Spread Pattern: A Case Report
- Carcinoma In Situ of the Urinary Bladder with Transitional Cell Carcinoma of Prostate: A Histopathologic Study and Mapping of the Urothelial Lesions
- Sarcomatoid urothelial carcinoma arising in the female urethral diverticulum
- CT Differentiation of Infiltrating Renal Cell Carcinoma and Renal Urothelial Tumor