J Breast Cancer.
2014 Mar;17(1):47-53.
Longer Survival in Patients with Breast Cancer with Cyclin D1 Over-Expression after Tumor Recurrence: Longer, but Occupied with Disease
- Affiliations
-
- 1Department of Surgery, Yonsei University Wonju College of Medicine, Wonju, Korea. airihan@yonsei.ac.kr
- 2Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Institute of Lifestyle Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
Abstract
- PURPOSE
The effect of cyclin D1 overexpression on breast cancer outcomes and prognosis is controversial, even though amplification of the cyclin D1 gene, CCND1, has been shown to be associated with early relapse and poor prognosis. In this study, we examined the relationship between cyclin D1 overexpression and disease-specific survival (DSS). We also analyzed survival in patients who experienced recurrence.
METHODS
We retrospectively analyzed data from patients diagnosed with ductal carcinoma between April 2005 and December 2010. We examined clinicopathologic factors associated with cyclin D1 overexpression and analyzed the influence of cyclin D1 on recurrence-free survival and DSS.
RESULTS
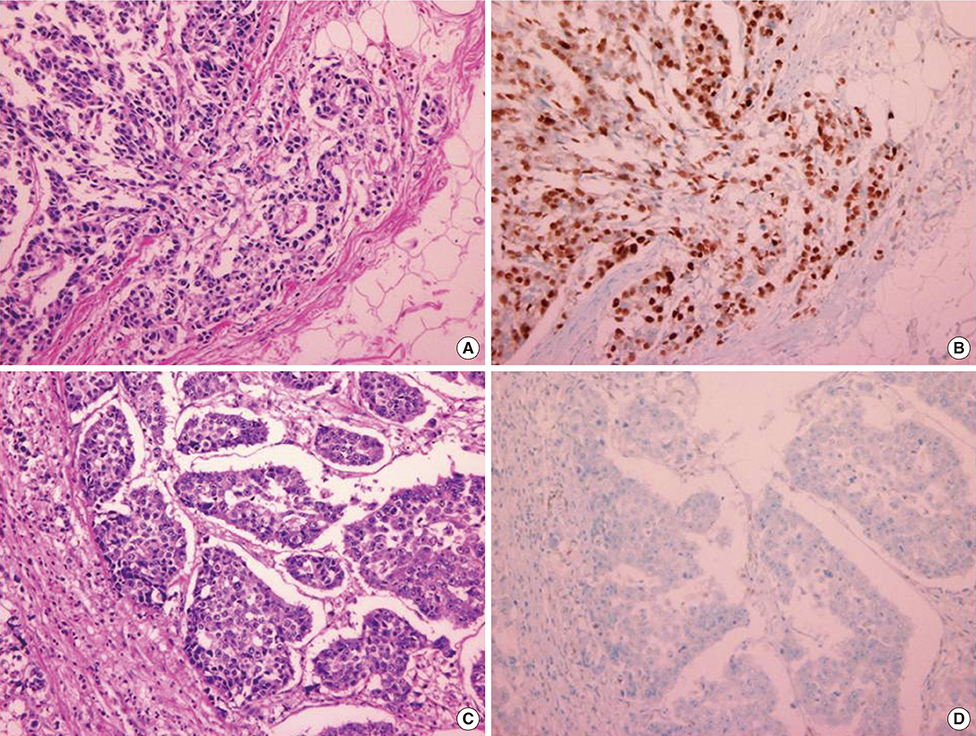
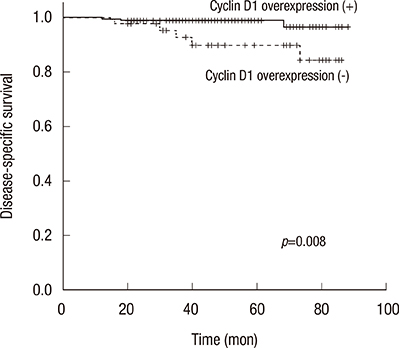
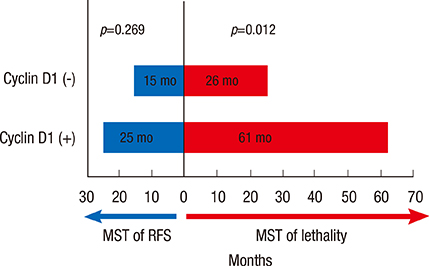
We identified 236 patients diagnosed with primary breast cancer who completed all phases of their primary treatment. Cyclin D1 overexpression was significantly associated with longer DSS (5-year DSS, 89.9% in patients without cyclin D1 overexpression vs. 98.9% in patients with cyclin D1 overexpression; p=0.008). Multivariate analysis also found that patients with cyclin D1 overexpressing tumors had significantly longer disease-specific survival than patients whose tumors did not overexpress cyclin D1, with a hazard ratio for disease-specific mortality of 7.97 (1.17-54.22, p=0.034). However, in the group of patients who experienced recurrence, cyclin D1 overexpression was not significantly associated with recurrence-free survival. Cyclin D1 overexpression was significantly associated with increased survival after disease recurrence, indicating that cyclin D1 overexpression might be indicative of more indolent disease progression after metastasis.
CONCLUSION
Cyclin D1 overexpression is associated with longer DSS, but not recurrence-free survival, in patients with breast cancer. Longer postrecurrence survival could explain the apparent inconsistency between DSS and recurrence-free survival. Patients with cyclin D1-overexpressing tumors survive longer, but with metastatic disease after recurrence. This information should spark the urgent development of tailored therapies to cure these patients.
MeSH Terms
Figure
Reference
-
1. Chang JC, Hilsenbeck SG. Prognostic and predictive markers. In : Harris JR, Lippman ME, Osborne CK, Morrow M, editors. Diseases of the Breast. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2010. p. 443–457.2. Lundgren K, Brown M, Pineda S, Cuzick J, Salter J, Zabaglo L, et al. Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Breast Cancer Res. 2012; 14:R57.
Article3. Roy PG, Thompson AM. Cyclin D1 and breast cancer. Breast. 2006; 15:718–727.
Article4. Velasco-Velázquez MA, Li Z, Casimiro M, Loro E, Homsi N, Pestell RG. Examining the role of cyclin D1 in breast cancer. Future Oncol. 2011; 7:753–765.
Article5. Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J Clin Oncol. 2005; 23:4215–4224.
Article6. Michalides R, Hageman P, van Tinteren H, Houben L, Wientjens E, Klompmaker R, et al. A clinicopathological study on overexpression of cyclin D1 and of p53 in a series of 248 patients with operable breast cancer. Br J Cancer. 1996; 73:728–734.
Article7. Stendahl M, Kronblad A, Rydén L, Emdin S, Bengtsson NO, Landberg G. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br J Cancer. 2004; 90:1942–1948.
Article8. Naidu R, Wahab NA, Yadav MM, Kutty MK. Expression and amplification of cyclin D1 in primary breast carcinomas: relationship with histopathological types and clinico-pathological parameters. Oncol Rep. 2002; 9:409–416.
Article9. McIntosh GG, Anderson JJ, Milton I, Steward M, Parr AH, Thomas MD, et al. Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene. 1995; 11:885–891.10. Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, Nicholson RI, et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res. 1999; 5:2069–2076.11. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999; 17:1474–1481.
Article12. Peurala E, Koivunen P, Haapasaari KM, Bloigu R, Jukkola-Vuorinen A. The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res. 2013; 15:R5.
Article13. Hwang TS, Han HS, Hong YC, Lee HJ, Paik NS. Prognostic value of combined analysis of cyclin D1 and estrogen receptor status in breast cancer patients. Pathol Int. 2003; 53:74–80.
Article14. Prall OW, Rogan EM, Musgrove EA, Watts CK, Sutherland RL. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Mol Cell Biol. 1998; 18:4499–4508.
Article15. Foster JS, Wimalasena J. Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol Endocrinol. 1996; 10:488–498.
Article16. Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker MG, Truss M, et al. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene. 1996; 12:2315–2324.17. Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997; 88:405–415.
Article18. Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998; 12:3488–3498.
Article19. Musgrove EA, Wakeling AE, Sutherland RL. Points of action of estrogen antagonists and a calmodulin antagonist within the MCF-7 human breast cancer cell cycle. Cancer Res. 1989; 49:2398–2404.20. Quintayo MA, Munro AF, Thomas J, Kunkler IH, Jack W, Kerr GR, et al. GSK3β and cyclin D1 expression predicts outcome in early breast cancer patients. Breast Cancer Res Treat. 2012; 136:161–168.
Article21. Caldon CE, Sutherland RL, Musgrove E. Cell cycle proteins in epithelial cell differentiation: implications for breast cancer. Cell Cycle. 2010; 9:1918–1928.
Article22. Lee A, Park WC, Yim HW, Lee MA, Park G, Lee KY. Expression of c-erbB2, cyclin D1 and estrogen receptor and their clinical implications in the invasive ductal carcinoma of the breast. Jpn J Clin Oncol. 2007; 37:708–714.
Article23. Lange CA, Yee D. Killing the second messenger: targeting loss of cell cycle control in endocrine-resistant breast cancer. Endocr Relat Cancer. 2011; 18:C19–C24.
Article24. Chen X, Bargonetti J, Prives C. p53, through p21 (WAF1/CIP1), induces cyclin D1 synthesis. Cancer Res. 1995; 55:4257–4263.25. Zukerberg LR, Yang WI, Gadd M, Thor AD, Koerner FC, Schmidt EV, et al. Cyclin D1 (PRAD1) protein expression in breast cancer: approximately one-third of infiltrating mammary carcinomas show overexpression of the cyclin D1 oncogene. Mod Pathol. 1995; 8:560–567.26. Nielsen NH, Lodén M, Cajander J, Emdin SO, Landberg G. G1-S transition defects occur in most breast cancers and predict outcome. Breast Cancer Res Treat. 1999; 56:105–112.
Article27. Shoker BS, Jarvis C, Davies MP, Iqbal M, Sibson DR, Sloane JP. Immunodetectable cyclin D(1)is associated with oestrogen receptor but not Ki67 in normal, cancerous and precancerous breast lesions. Br J Cancer. 2001; 84:1064–1069.
Article28. de Jong JS, van Diest PJ, Michalides RJ, Baak JP. Concerted overexpression of the genes encoding p21 and cyclin D1 is associated with growth inhibition and differentiation in various carcinomas. Mol Pathol. 1999; 52:78–83.
Article29. Nik-Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, et al. The life history of 21 breast cancers. Cell. 2012; 149:994–1007.
Article30. Martins FC, De S, Almendro V, Gönen M, Park SY, Blum JL, et al. Evolutionary pathways in BRCA1-associated breast tumors. Cancer Discov. 2012; 2:503–511.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Cyclin D1 Protein in Breast Cancer and Its Correlation with Prognosis
- Expression of Cyclin D1 and bcl-2 in Infiltrative Ductal Carcinoma of the Breast: Their Correlations and Clinical implications
- Significance of Expression of bcl-2, p53 and cyclin D1 and Their Correlation with Clinicopathologic Prognostic Factors and Survival Rate in 128 Cases of Invasive Breast Carcinoma
- Cyclin D1 Expression and Patient Outcome After Tamoxifen Therapy in Estrogen Receptor Positive Breast Cancer
- Study of the Relationships between Cyclin D1 and Known Prognostic Factors in Breast Cancer