Korean J Radiol.
2016 Feb;17(1):83-92. 10.3348/kjr.2016.17.1.83.
Coronary Microembolization with Normal Epicardial Coronary Arteries and No Visible Infarcts on Nitrobluetetrazolium Chloride-Stained Specimens: Evaluation with Cardiac Magnetic Resonance Imaging in a Swine Model
- Affiliations
-
- 1Department of Radiology, Zhongshan Hospital, Fudan University and Shanghai Institute of Medical Imaging, Shanghai 200032, China. zeng.mengsu@hotmail.com
- 2Department of Medical Imaging, Shanghai Medical College, Fudan University, Shanghai 200032, China.
- 3Department of Cardiology, Zhongshan Hospital, Fudan University and Shanghai Institute of Cardiovascular Diseases, Shanghai 200032, China.
- KMID: 2351167
- DOI: http://doi.org/10.3348/kjr.2016.17.1.83
Abstract
OBJECTIVE
To assess magnetic resonance imaging (MRI) features of coronary microembolization in a swine model induced by small-sized microemboli, which may cause microinfarcts invisible to the naked eye.
MATERIALS AND METHODS
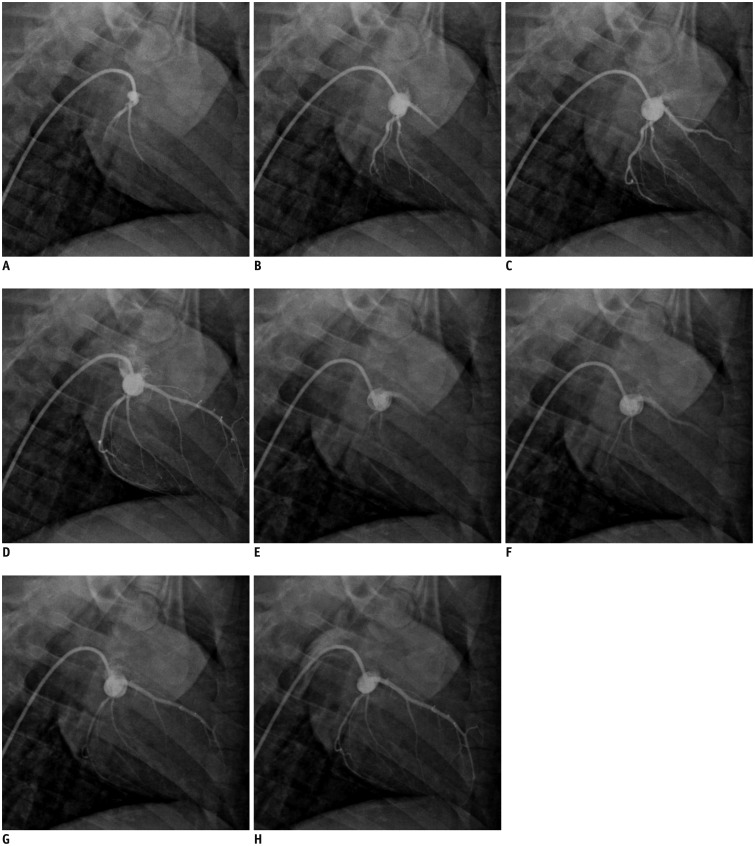
Eleven pigs underwent intracoronary injection of small-sized microspheres (42 microm) and catheter coronary angiography was obtained before and after microembolization. Cardiac MRI and measurement of cardiac troponin T (cTnT) were performed at baseline, 6 hours, and 1 week after microembolization. Postmortem evaluation was performed after completion of the imaging studies.
RESULTS
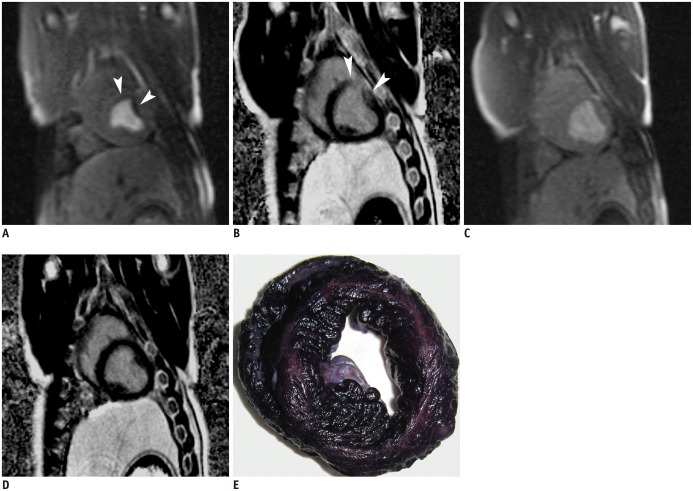
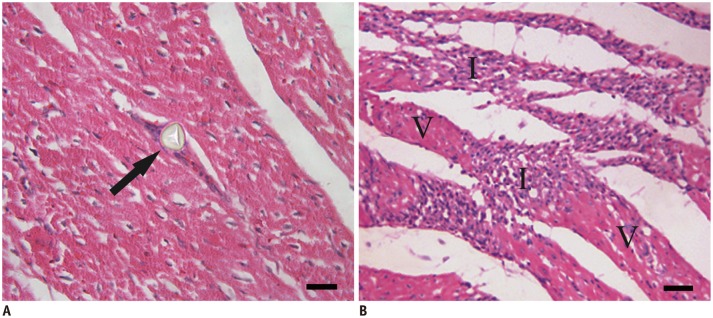
Coronary angiography pre- and post-microembolization revealed normal epicardial coronary arteries. Systolic wall thickening of the microembolized regions decreased significantly from 42.6 +/- 2.0% at baseline to 20.3 +/- 2.3% at 6 hours and 31.5 +/- 2.1% at 1 week after coronary microembolization (p < 0.001 for both). First-pass perfusion defect was visualized at 6 hours but the extent was largely decreased at 1 week. Delayed contrast enhancement MRI (DE-MRI) demonstrated hyperenhancement within the target area at 6 hours but not at 1 week. The microinfarcts on gross specimen stained with nitrobluetetrazolium chloride were invisible to the naked eye and only detectable microscopically. Increased cTnT was observed at 6 hours and 1 week after microembolization.
CONCLUSION
Coronary microembolization induced by a certain load of small-sized microemboli may result in microinfarcts invisible to the naked eye with normal epicardial coronary arteries. MRI features of myocardial impairment secondary to such microembolization include the decline in left ventricular function and myocardial perfusion at cine and first-pass perfusion imaging, and transient hyperenhancement at DE-MRI.
MeSH Terms
-
Animals
Coronary Angiography/*methods
Coronary Vessels/*pathology
Disease Models, Animal
Embolism/*pathology
Female
Heart/radiography
Image Processing, Computer-Assisted
Magnetic Resonance Imaging/*methods
Microspheres
Myocardial Contraction/physiology
Myocardial Infarction/*pathology
Myocardium/pathology
Nitroblue Tetrazolium
Staining and Labeling
Swine
Troponin T/blood
Ventricular Function, Left
Nitroblue Tetrazolium
Troponin T
Figure
Reference
-
1. Porto I, Selvanayagam JB, Van Gaal WJ, Prati F, Cheng A, Channon K, et al. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation. 2006; 114:662–669. PMID: 16894040.2. Baumgart D, Liu F, Haude M, Görge G, Ge J, Erbel R. Acute plaque rupture and myocardial stunning in patient with normal coronary arteriography. Lancet. 1995; 346:193–194. PMID: 7603266.
Article3. Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation. 1985; 71:699–708. PMID: 3971539.
Article4. Kotani J, Nanto S, Mintz GS, Kitakaze M, Ohara T, Morozumi T, et al. Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation. 2002; 106:1672–1677. PMID: 12270861.
Article5. Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, van't Hof AW, Hoorntje JC, et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002; 23:1112–1117. PMID: 12090749.
Article6. Selvanayagam JB, Cheng AS, Jerosch-Herold M, Rahimi K, Porto I, van Gaal W, et al. Effect of distal embolization on myocardial perfusion reserve after percutaneous coronary intervention: a quantitative magnetic resonance perfusion study. Circulation. 2007; 116:1458–1464. PMID: 17785626.7. Dörge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, et al. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 2000; 279:H2587–H2592. PMID: 11087208.
Article8. Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000; 101:570–580. PMID: 10662756.
Article9. Skyschally A, Leineweber K, Gres P, Haude M, Erbel R, Heusch G. Coronary microembolization. Basic Res Cardiol. 2006; 101:373–382. PMID: 16915530.
Article10. Jeremy RW, Links JM, Becker LC. Progressive failure of coronary flow during reperfusion of myocardial infarction: documentation of the no reflow phenomenon with positron emission tomography. J Am Coll Cardiol. 1990; 16:695–704. PMID: 2387943.
Article11. Asanuma T, Tanabe K, Ochiai K, Yoshitomi H, Nakamura K, Murakami Y, et al. Relationship between progressive microvascular damage and intramyocardial hemorrhage in patients with reperfused anterior myocardial infarction: myocardial contrast echocardiographic study. Circulation. 1997; 96:448–453. PMID: 9244211.12. Gerber BL, Rochitte CE, Melin JA, McVeigh ER, Bluemke DA, Wu KC, et al. Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation. 2000; 101:2734–2741. PMID: 10851212.
Article13. Kaul S, Ito H. Microvasculature in acute myocardial ischemia: part II: evolving concepts in pathophysiology, diagnosis, and treatment. Circulation. 2004; 109:310–315. PMID: 14744956.14. Litchfield RL. Noninvasive tests for cardiac risk stratification. Which ones are most prognostic? Postgrad Med. 2004; 115:30–36. PMID: 15000058.15. Poon M, Fuster V, Fayad Z. Cardiac magnetic resonance imaging: a "one-stop-shop" evaluation of myocardial dysfunction. Curr Opin Cardiol. 2002; 17:663–670. PMID: 12466710.
Article16. Finn JP, Nael K, Deshpande V, Ratib O, Laub G. Cardiac MR imaging: state of the technology. Radiology. 2006; 241:338–354. PMID: 17057063.
Article17. Sakuma H, Ichikawa Y, Suzawa N, Hirano T, Makino K, Koyama N, et al. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology. 2005; 237:316–321. PMID: 16126921.
Article18. Jin H, Zeng MS, Ge MY, Ma JY, Chen CZ, Shen JZ, et al. Influence of applying nitroglycerin in whole-heart free-breathing 3D coronary MR angiography. AJR Am J Roentgenol. 2010; 194:927–932. PMID: 20308493.
Article19. Yun H, Zeng MS, Jin H, Yang S. Isolated noncompaction of ventricular myocardium: a magnetic resonance imaging study of 11 patients. Korean J Radiol. 2011; 12:686–692. PMID: 22043150.
Article20. Carlsson M, Wilson M, Martin AJ, Saeed M. Myocardial microinfarction after coronary microembolization in swine: MR imaging characterization. Radiology. 2009; 250:703–713. PMID: 19164123.
Article21. Carlsson M, Martin AJ, Ursell PC, Saloner D, Saeed M. Magnetic resonance imaging quantification of left ventricular dysfunction following coronary microembolization. Magn Reson Med. 2009; 61:595–602. PMID: 19097239.
Article22. Carlsson M, Jablonowski R, Martin AJ, Ursell PC, Saeed M. Coronary microembolization causes long-term detrimental effects on regional left ventricular function. Scand Cardiovasc J. 2011; 45:205–214. PMID: 21463182.
Article23. Carlsson M, Saloner D, Martin AJ, Ursell PC, Saeed M. Heterogeneous microinfarcts caused by coronary microemboli: evaluation with multidetector CT and MR imaging in a swine model. Radiology. 2010; 254:718–728. PMID: 20177087.
Article24. Möhlenkamp S, Beighley PE, Pfeifer EA, Behrenbeck TR, Sheedy PF 2nd, Ritman EL. Intramyocardial blood volume, perfusion and transit time in response to embolization of different sized microvessels. Cardiovasc Res. 2003; 57:843–852. PMID: 12618246.25. Saeed M, Hetts SW, Do L, Sullivan SM, Wilson MW. MRI quantification of left ventricular function in microinfarct versus large infarct in swine model. Int J Cardiovasc Imaging. 2013; 29:159–168. PMID: 23065097.
Article26. Breuckmann F, Nassenstein K, Bucher C, Konietzka I, Kaiser G, Konorza T, et al. Systematic analysis of functional and structural changes after coronary microembolization: a cardiac magnetic resonance imaging study. JACC Cardiovasc Imaging. 2009; 2:121–130. PMID: 19356544.
Article27. Rodrigues de Avila LF, Fernandes JL, Rochitte CE, Cerri GG, Filho JP. Perfusion impairment in patients with normal-appearing coronary arteries: identification with contrast-enhanced MR imaging. Radiology. 2006; 238:464–472. PMID: 16371584.
Article28. Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002; 47:372–383. PMID: 11810682.
Article29. Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment--freely available software for cardiovascular image analysis. BMC Med Imaging. 2010; 10:1. PMID: 20064248.
Article30. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.
Article31. Edelman RR. Contrast-enhanced MR imaging of the heart: overview of the literature. Radiology. 2004; 232:653–668. PMID: 15284429.
Article32. Skyschally A, Haude M, Dörge H, Thielmann M, Duschin A, van de Sand A, et al. Glucocorticoid treatment prevents progressive myocardial dysfunction resulting from experimental coronary microembolization. Circulation. 2004; 109:2337–2342. PMID: 15117838.
Article33. Huber AM, Schoenberg SO, Hayes C, Spannagl B, Engelmann MG, Franz WM, et al. Phase-sensitive inversion-recovery MR imaging in the detection of myocardial infarction. Radiology. 2005; 237:854–860. PMID: 16304107.
Article34. Jiang L, Huang Y, Hunyor S, dos Remedios CG. Cardiomyocyte apoptosis is associated with increased wall stress in chronic failing left ventricle. Eur Heart J. 2003; 24:742–751. PMID: 12713768.
Article35. Chen ZW, Qian JY, Ma JY, Chang SF, Yun H, Jin H, et al. TNF-α-induced cardiomyocyte apoptosis contributes to cardiac dysfunction after coronary microembolization in mini-pigs. J Cell Mol Med. 2014; 18:1953–1963. PMID: 25130514.36. De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but "Normal" coronary angiography. Circulation. 2001; 104:2401–2406. PMID: 11705815.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiac MR Assessment of Coronary Arteries
- Coronary MDCT and MRI
- Troponin-Positive Non-Obstructive Coronary Arteries and Myocardial Infarction with Non-Obstructive Coronary Arteries: Definition, Etiologies, and Role of CT and MR Imaging
- Additive Role of Coronary Magnetic Resonance Angiography for the Evaluation of Coronary Artery Disease
- Assessment of Patency of Coronary Artery Bypass Grafts Using Segmented K-space Breath-hold Cine Cardiovascular Magnetic Resonance Imaging: A Clinical Feasibility Study