J Korean Med Sci.
2015 Nov;30(11):1689-1697. 10.3346/jkms.2015.30.11.1689.
Reproducibility of Apparent Diffusion Coefficient Measurements in Malignant Breast Masses
- Affiliations
-
- 1Department of Radiology, Seoul National University Bundang Hospital, Seongnam, Korea. kimsmlms@daum.net
- 2Department of Radiology, Chung-Ang University Hospital, Seoul, Korea.
- 3Department of Radiology, Hanyang University Guri Hospital, Guri, Korea.
- 4Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Department of Surgery, Breast Care Center, Daerim St. Mary's Hospital, Seoul, Korea.
- KMID: 2351122
- DOI: http://doi.org/10.3346/jkms.2015.30.11.1689
Abstract
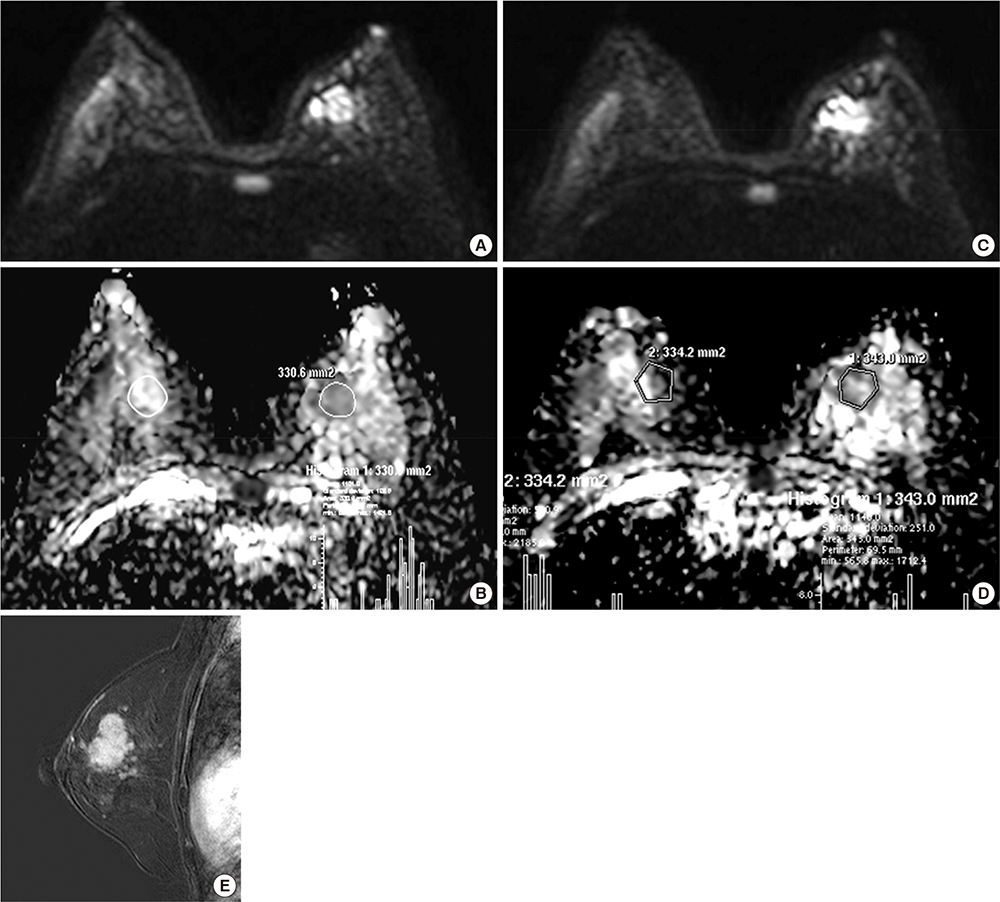
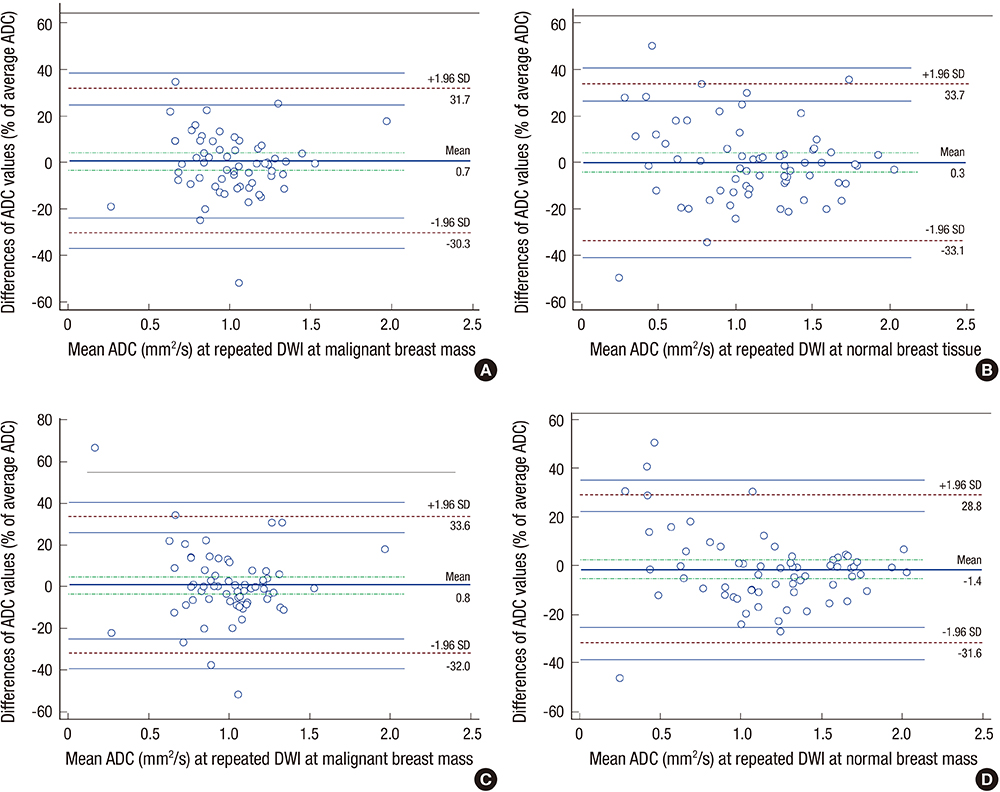
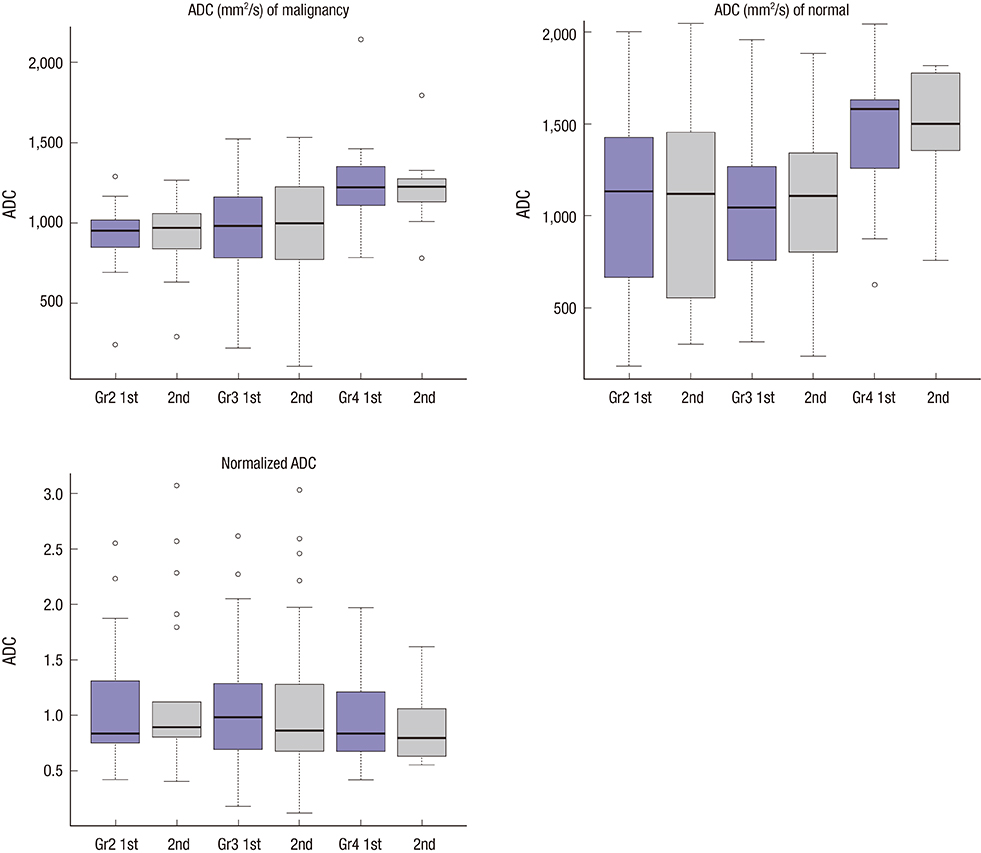
- This study aimed to evaluate the reproducibility of apparent diffusion coefficient (ADC) measurements in malignant breast masses, and to determine the influence of mammographic parenchymal density on this reproducibility. Sixty-six patients with magnetic resonance findings of the mass were included. Two breast radiologists measured the ADC of the malignant breast mass and the same area on the contralateral normal breast in each patient twice. The effects of mammographic parenchymal density, histology, and lesion size on reproducibility were also assessed. There was no significant difference in the mean ADC between repeated measurements in malignant breast masses and normal breast tissue. The overall reproducibility of ADC measurements was good in both. The 95% limits of agreement for repeated ADCs were approximately 30.2%-33.4% of the mean. ADC measurements in malignant breast masses were highly reproducible irrespective of mass size, histologic subtype, or coexistence of microcalcifications; however, the measurements tended to be less reproducible in malignant breast masses with extremely dense parenchymal backgrounds. ADC measurements in malignant breast masses are highly reproducible; however, mammographic parenchymal density can potentially influence this reproducibility.
Keyword
MeSH Terms
Figure
Reference
-
1. Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009; 11:102–125.2. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007; 188:1622–1635.3. Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007; 25:4104–4109.4. Woodhams R, Ramadan S, Stanwell P, Sakamoto S, Hata H, Ozaki M, Kan S, Inoue Y. Diffusion-weighted imaging of the breast: principles and clinical applications. Radiographics. 2011; 31:1059–1084.5. Herneth AM, Guccione S, Bednarski M. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol. 2003; 45:208–213.6. Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, Mahankali S, Gao JH. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002; 16:172–178.7. Park MJ, Cha ES, Kang BJ, Ihn YK, Baik JH. The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol. 2007; 8:390–396.8. Woodhams R, Matsunaga K, Kan S, Hata H, Ozaki M, Iwabuchi K, Kuranami M, Watanabe M, Hayakawa K. ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci. 2005; 4:35–42.9. Yoshikawa MI, Ohsumi S, Sugata S, Kataoka M, Takashima S, Mochizuki T, Ikura H, Imai Y. Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat Med. 2008; 26:222–226.10. Park SH, Moon WK, Cho N, Song IC, Chang JM, Park IA, Han W, Noh DY. Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology. 2010; 257:56–63.11. Nasu K, Kuroki Y, Fujii H, Minami M. Hepatic pseudo-anisotropy: a specific artifact in hepatic diffusion-weighted images obtained with respiratory triggering. MAGMA. 2007; 20:205–211.12. Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging. 2006; 24:478–488.13. Kim SY, Lee SS, Byun JH, Park SH, Kim JK, Park B, Kim N, Lee MG. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology. 2010; 255:815–823.14. Kwee TC, Takahara T, Koh DM, Nievelstein RA, Luijten PR. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J Magn Reson Imaging. 2008; 28:1141–1148.15. Nasu K, Kuroki Y, Sekiguchi R, Kazama T, Nakajima H. Measurement of the apparent diffusion coefficient in the liver: is it a reliable index for hepatic disease diagnosis? Radiat Med. 2006; 24:438–444.16. Bogner W, Pinker-Domenig K, Bickel H, Chmelik M, Weber M, Helbich TH, Trattnig S, Gruber S. Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology. 2012; 263:64–76.17. Kim YJ, Kim SH, Kang BJ, Park CS, Kim HS, Son YH, Porter DA, Song BJ. Readout-segmented echo-planar imaging in diffusion-weighted mr imaging in breast cancer: comparison with single-shot echo-planar imaging in image quality. Korean J Radiol. 2014; 15:403–410.18. O'Flynn EA, Morgan VA, Giles SL, deSouza NM. Diffusion weighted imaging of the normal breast: reproducibility of apparent diffusion coefficient measurements and variation with menstrual cycle and menopausal status. Eur Radiol. 2012; 22:1512–1518.19. Ei Khouli RH, Jacobs MA, Mezban SD, Huang P, Kamel IR, Macura KJ, Bluemke DA. Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging. Radiology. 2010; 256:64–73.20. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979; 86:420–428.21. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–310.22. Partridge SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol. 2009; 193:1716–1722.23. Rubesova E, Grell AS, De Maertelaer V, Metens T, Chao SL, Lemort M. Quantitative diffusion imaging in breast cancer: a clinical prospective study. J Magn Reson Imaging. 2006; 24:319–324.24. Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008; 246:116–124.25. Bogner W, Gruber S, Pinker K, Grabner G, Stadlbauer A, Weber M, Moser E, Helbich TH, Trattnig S. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis. Radiology. 2009; 253:341–351.26. Pereira FP, Martins G, Figueiredo E, Domingues MN, Domingues RC, da Fonseca LM, Gasparetto EL. Assessment of breast lesions with diffusion-weighted MRI: comparing the use of different b values. AJR Am J Roentgenol. 2009; 193:1030–1035.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Weighted Imaging for Differentiating Benign from Malignant Orbital Tumors: Diagnostic Performance of the Apparent Diffusion Coefficient Based on Region of Interest Selection Method
- Reversal of a Large Ischemic Lesion with Low Apparent Diffusion Coefficient Value by Rapid Spontaneous Recanalization
- Usefulness of Diffusion-Weighted MR Imaging for Breast Lesions: Comparing the Apparent Diffusion Coefficient (ADC) Values and the Pathologic Results
- Clinical applications and characteristics of apparent diffusion coefficient maps for the brain of two dogs
- Imaging Findings of Pancreatic Solid Pseudopapillary Neoplasm with High-Grade Malignant Transformation: Focusing on Diffusion-Weighted Imaging and Normalized Apparent Diffusion Coefficient Values