Allergy Asthma Respir Dis.
2016 Jul;4(4):301-304. 10.4168/aard.2016.4.4.301.
Methazolamide-induced toxic epidermal necrolysis confirmed by lymphocyte activation test
- Affiliations
-
- 1Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea. ykjee@dankook.ac.kr
- 2Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2346761
- DOI: http://doi.org/10.4168/aard.2016.4.4.301
Abstract
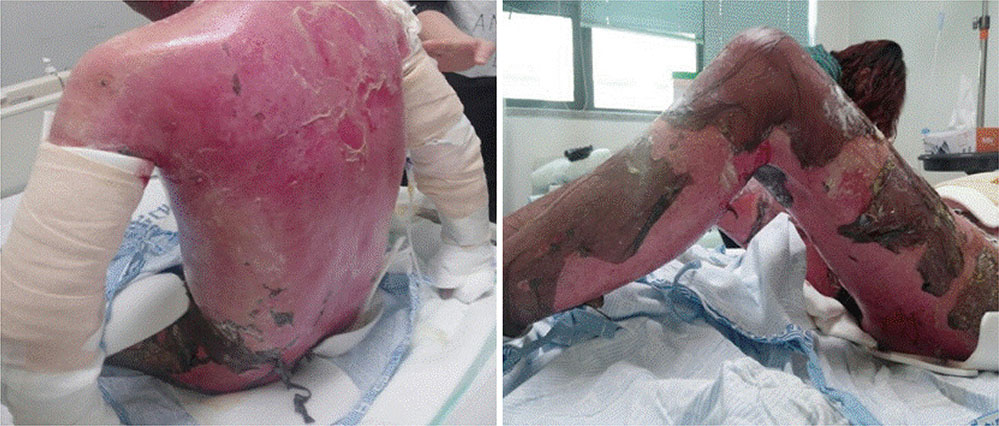
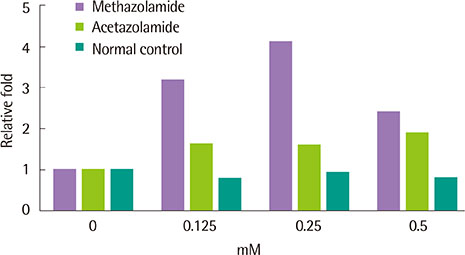
- Among various dermatological entities, toxic epidermal necrolysis (TEN) is a rare but potentially fatal delayed hypersensitivity reaction to numerous medications. A 38-year-old male presented with systemic hypersensitivity reaction, such as high fever, pain in the eyes, and diffuse pruritic erythematous maculopapular eruptions with multiple targetoid plaques that became vesicular and bullous. Oral mucosa and conjunctivae were involved. The first sign appeared about 1 week after taking methazolamide (50 mg twice a day) for the management of glaucomatous eyes. Although methazolamide was discontinued, blistering and skin denudation progressed to affect up to 80% of the body surface area and a positive Nikolsky sign was noted. High fever also persisted. Skin lesions started to improve after 2 weeks of management and fever subsided. Cutaneous lesions improved with minimal permanent sequele 2 months later. HLA-B*5901 was found by high-resolution genotyping. The lymphocyte activation test performed 6 months after remission showed a positive response to methazolamide challenge. This is the first case of methazolamide-induced TEN in which methazolamide was confirmed as a culprit drug by the lymphocyte activation test.
MeSH Terms
Figure
Reference
-
1. Maren TH, Haywood JR, Chapman SK, Zimmerman TJ. The pharmacology of methazolamide in relation to the treatment of glaucoma. Invest Ophthalmol Vis Sci. 1977; 16:730–742.2. Kim SH, Kim M, Lee KW, Kim SH, Kang HR, Park HW, et al. HLA-B*5901 is strongly associated with methazolamide-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Pharmacogenomics. 2010; 11:879–884.
Article3. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004; 59:809–820.
Article4. Seo YS, Chang KC, Chang MH. Methazolamide-induced Stevens-Johnson Syndrome. J Korean Ophthalmol Soc. 2009; 50:1881–1886.
Article5. Schopf E, Mockenhaupt M. Drug-attributability in severe skin reactions: toxic epidermal necrolysis (TEN) and Stevens-Johnson Syndrome (SJS). Korean J Dermatol. 1999; 37:975–982.6. Raviglione MC, Pablos-Mendez A, Battan R. Clinical features and management of severe dermatological reactions to drugs. Drug Saf. 1990; 5:39–64.
Article7. Dunagin WG, Milikan LE. Drug eruptions. Med Clin North Am. 1980; 64:983–1003.
Article8. Genvert GI, Cohen EJ, Donnenfeld ED, Blecher MH. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985; 99:465–468.
Article9. Roujeau JC, Huynh TN, Bracq C, Guillaume JC, Revuz J, Touraine R. Genetic susceptibility to toxic epidermal necrolysis. Arch Dermatol. 1987; 123:1171–1173.
Article10. Imanishi T, Akaza T, Kimura A, Tokunaga K, Gojobori T. Allele and haplotype frequencies for HLA and complement loci in various ethnic groups. In : Tshuji K, Aizawa M, Sasazuki T, editors. HLA 1991: proceedings of the eleventh International Histocompatibility Workshop and Conference; 1991 Nov 6-13; Yokohama, Japan. New York: Oxford University Press;1991. p. 1065–1220.11. Yang F, Xuan J, Chen J, Zhong H, Luo H, Zhou P, et al. HLA-B*59:01: a marker for Stevens-Johnson syndrome/toxic epidermal necrolysis caused by methazolamide in Han Chinese. Pharmacogenomics J. 2016; 16:83–87.
Article12. Lee KW, Oh DH, Lee C, Yang SY. Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 2005; 65:437–447.
Article13. Saito S, Ota S, Yamada E, Inoko H, Ota M. Allele frequencies and haplotypic associations defined by allelic DNA typing at HLA class I and class II loci in the Japanese population. Tissue Antigens. 2000; 56:522–529.
Article14. Chung WH, Hung SI, Chen YT. Human leukocyte antigens and drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2007; 7:317–323.
Article15. Alfirevic A, Jorgensen AL, Williamson PR, Chadwick DW, Park BK, Pirmohamed M. HLA-B locus in Caucasian patients with carbamazepine hypersensitivity. Pharmacogenomics. 2006; 7:813–818.
Article16. Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008; 9:1617–1622.
Article17. Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011; 364:1126–1133.
Article18. Wolkenstein P, Chosidow O, Fléchet ML, Robbiola O, Paul M, Dume L, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996; 35:234–236.
Article19. Moon JI, Seo JH, Park CK. Association of HLA type with Stevens-Johnson syndrome induced by methazolamide treatment. J Korean Ophthalmol Soc. 2000; 41:2241–2246.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of HLA-B59(+) Stevens-Johnson Syndrome (SJS)-Toxic Epidermal Necrolysis (TEN) Associated with Methazolamide Treatment
- Epidermal Necrolysis due to Methazolamide Treatment in Glaucomatous Patients
- Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome Caused by Topical Ophthalmic Use of Dorzolamide
- A Case of Carbamazepine- Induced- Toxic Epidermal Necrolysis
- Methazolamide-induced Stevens-Johnson Syndrome