Korean Circ J.
2016 Mar;46(2):197-206. 10.4070/kcj.2016.46.2.197.
Early Parasympathetic Reinnervation Is Not Related to Reconnection of Major Branches of the Vagus Nerve after Heart Transplantation
- Affiliations
-
- 1Department of Internal Medicine, Division of Cardiology, Seoul National University Hospital, Seoul, Korea. seil@snu.ac.kr
- KMID: 2344471
- DOI: http://doi.org/10.4070/kcj.2016.46.2.197
Abstract
- BACKGROUND AND OBJECTIVES
Bicaval heart transplantation (HTx) may promote parasympathetic reinnervation. However, the prevalence and timing of reinnervation have not been fully investigated. Heart rate variability (HRV) and direct vagal stimulation were used to evaluate the presence of parasympathetic reinnervation after bicaval HTx.
SUBJECTS AND METHODS
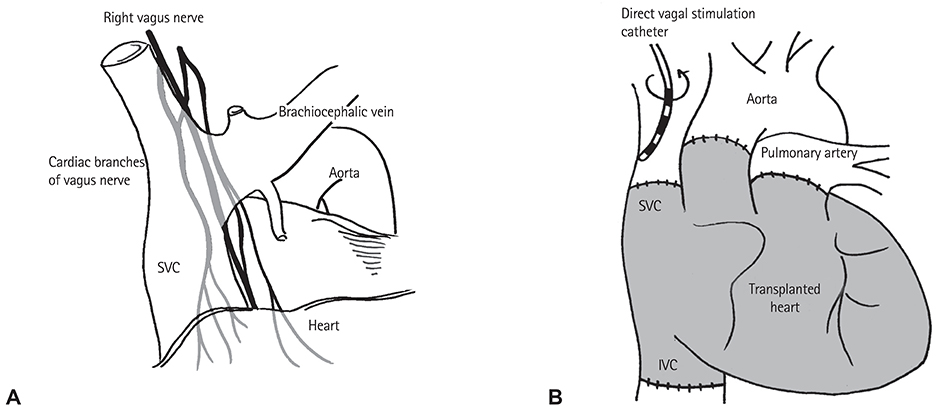
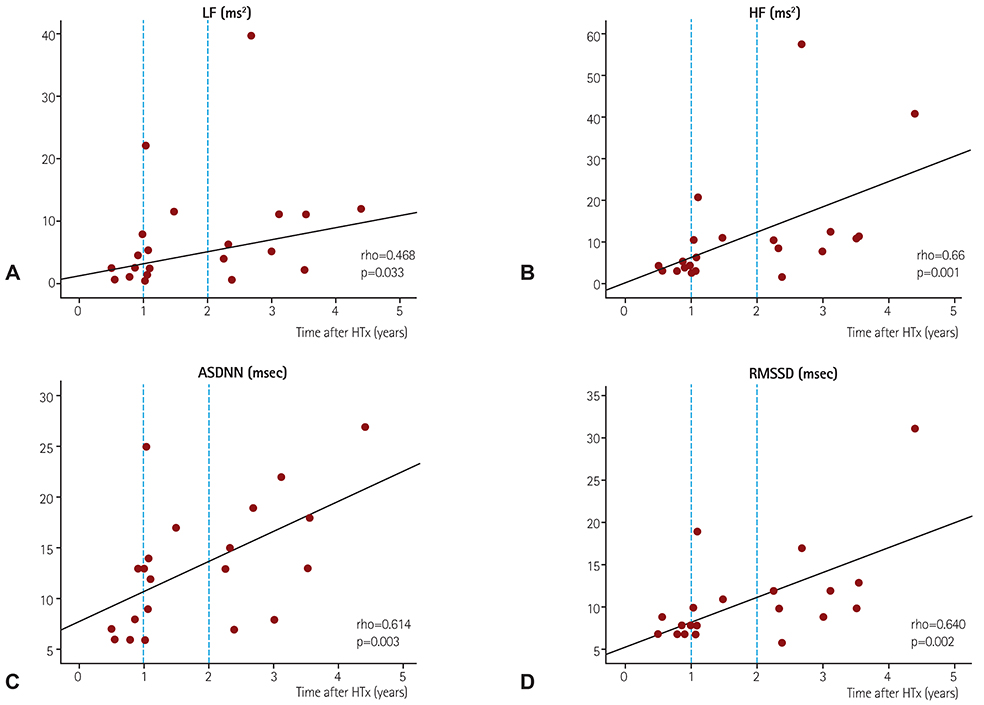
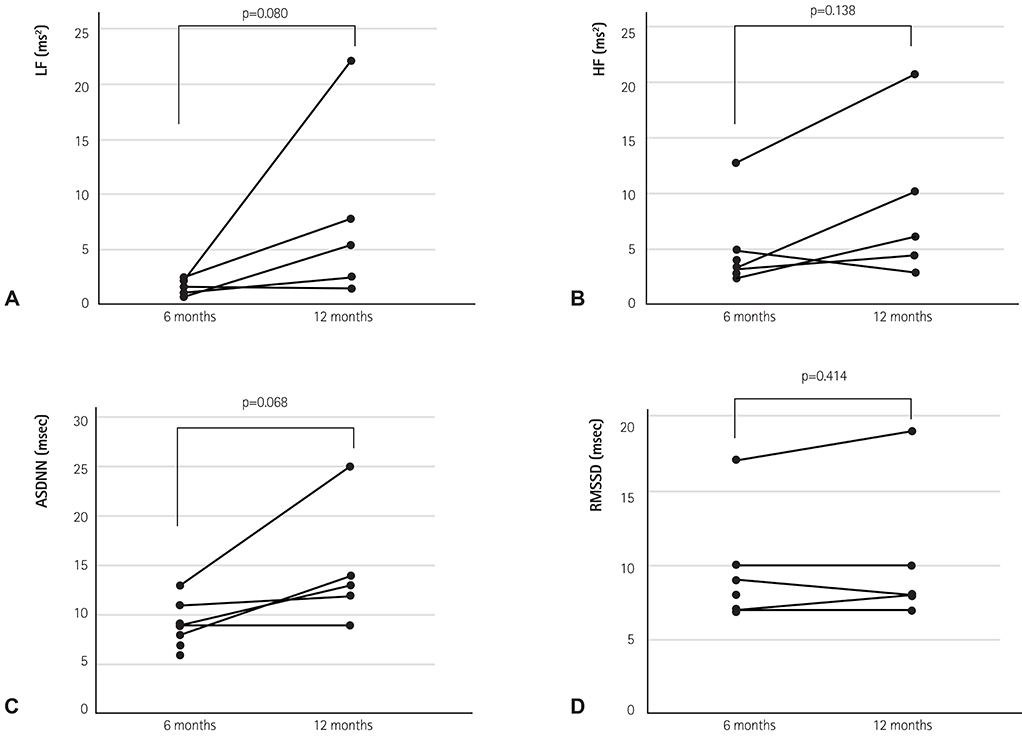
A total of 21 patients (time after HTx 0.52-4.41 years, mean 1.8±1.2 years) who received a bicaval HTx was enrolled. Reinnervation was evaluated using HRV values from 24-hour Holter recordings. A cross-sectional analysis of the HRV at 0.5-1, 1-2, and >2 years after HTx was performed. We also applied high-frequency electrical stimulation (16.7 Hz, 1 msec pulse width, ≤10 V) to the cardiac branches of the vagus nerve at the level of the superior vena cava in eight patients at 6 and 12 months after HTx.
RESULTS
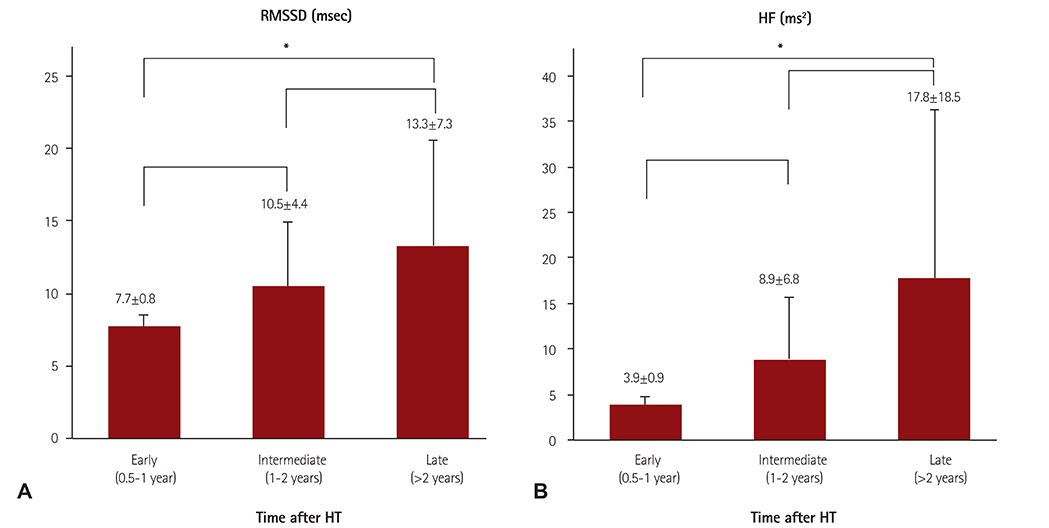
The degree of parasympathetic reinnervation corresponded to the time after HTx. The HRV analysis revealed that the root mean square of the successive differences between consecutive RR-intervals (RMSSD) and high-frequency power were significantly higher during the late period (>2 years) compared with the early period (0.5-1 year) after HTx. None of the eight patients who underwent direct vagal stimulation responded during the stimulation at 6 and 12 months, whereas incremental trends in HRV parameters were observed, which indicated that parasympathetic reinnervation began within 1 year after HTx.
CONCLUSION
Parasympathetic reinnervation seemed to begin in the early period (<1 year) after bicaval HTx. Reconnection of major branches of the vagus nerve may not be related to early reinnervation.
MeSH Terms
Figure
Reference
-
1. Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med. 2001; 345:731–738.2. Kleiger RE, Miller JP, Bigger JT JR, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987; 59:256–262.3. Imamura T, Kinugawa K, Okada I, et al. Parasympathetic reinnervation accompanied by improved post-exercise heart rate recovery and quality of life in heart transplant recipients. Int Heart J. 2015; 56:180–185.4. Bengel FM, Ueberfuhr P, Ziegler SI, Nekolla S, Reichart B, Schwaiger M. Serial assessment of sympathetic reinnervation after orthotopic heart transplantation. A longitudinal study using PET and C-11 hydroxyephedrine. Circulation. 1999; 99:1866–1871.5. Buendia-Fuentes F, Almenar L, Ruiz C, et al. Sympathetic reinnervation 1 year after heart transplantation, assessed using iodine-123 metaiodobenzylguanidine imaging. Transplant Proc. 2011; 43:2247–2248.6. Halpert I, Goldberg AD, Levine AB, et al. Reinnervation of the transplanted human heart as evidenced from heart rate variability studies. Am J Cardiol. 1996; 77:180–183.7. Wilson RF, Christensen BV, Olivari MT, Simon A, White CW, Laxson DD. Evidence for structural sympathetic reinnervation after orthotopic cardiac transplantation in humans. Circulation. 1991; 83:1210–1220.8. Schwaiger M, Hutchins GD, Kalff V, et al. Evidence for regional catecholamine uptake and storage sites in the transplanted human heart by positron emission tomography. J Clin Invest. 1991; 87:1681–1690.9. Roper S, Taylor B. Reinnervation of denervated parasympathetic neurons in cardiac ganglia from Rana pipiens. J Physiol. 1982; 326:155–171.10. Kondo Y, Matheny JL, Hardy JD. Autonomic reinnervation of cardiac transplants: further observations in dogs and rhesus monkeys. Ann Surg. 1972; 176:42–48.11. Raczak G, La Rovere MT, Mortara A, et al. Arterial baroreflex modulation of heart rate in patients early after heart transplantation: lack of parasympathetic reinnervation. J Heart Lung Transplant. 1999; 18:399–406.12. Keeley EC, Toth ZK, Goldberg AD. Long-term assessment of heart rate variability in cardiac transplant recipients. J Heart Lung Transplant. 2000; 19:310–312.13. Bernardi L, Valenti C, Wdowczyck-Szulc J, et al. Influence of type of surgery on the occurrence of parasympathetic reinnervation after cardiac transplantation. Circulation. 1998; 97:1368–1374.14. Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991; 84:482–492.15. Uberfuhr P, Frey AW, Fuchs A, et al. Signs of vagal reinnervation 4 years after heart transplantation in spectra of heart rate variability. Eur J Cardiothorac Surg. 1997; 12:907–912.16. Tio RA, Reyners AK, van Veldhuisen DJ, et al. Evidence for differential sympathetic and parasympathetic reinnervation after heart transplantation in humans. J Auton Nerv Syst. 1997; 67:176–183.17. Cornelissen VA, Vanhaecke J, Aubert AE, Fagard RH. Heart rate variability after heart transplantation: a 10-year longitudinal follow-up study. J Cardiol. 2012; 59:220–224.18. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000; 284:3043–3045.19. Ewing DJ. Analysis of heart rate variability and other non-invasive tests with special reference to diabetes mellitus. In : Bannister R, Mathias CJ, editors. Autonomic Failure. A textbook of clinical disorders of the autonomic nervous system. 3rd ed. Oxford: Oxford University Press;1992. p. 312–333.20. Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985; 249(4 Pt 2):H867–H875.21. Keith LM, Arther FD. Clinically oriented anatomy. 4th ed. Baltimore: Lippincott Williams & Wilkins;1992. p. 137–138.22. Murphy DA, Thompson GW, Ardell JL, et al. The heart reinnervates after transplantation. Ann Thorac Surg. 2000; 69:1769–1781.23. Rowan RA, Billingham ME. Myocardial innervation in long-term heart transplant survivors: a quantitative ultrastructural survey. J Heart Transplant. 1988; 7:448–452.24. Gallego-Page JC, Segovia J, Alonso-Pulpón L, Alonso-Rodríguez M, Salas C, Ortíz-Berrocal J. Re-innervation after heart transplantation: a multidisciplinary study. J Heart Lung Transplant. 2004; 23:674–682.25. Bilchick KC, Berger RD. Heart rate variability. J Cardiovasc Electrophysiol. 2006; 17:691–694.26. Breuer HW, Skyschally A, Schulz R, Martin C, Wehr M, Heusch G. Heart rate variability and circulating catecholamine concentrations during steady state exercise in healthy volunteers. Br Heart J. 1993; 70:144–149.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A rare variation of the glossopharyngeal nerve
- Neurilemmomas of the Cervicla Vagus Nerve: A Case Report
- Videofluoroscopy-Guided Balloon Dilatation for the Opening Dysfunction of Upper Esophageal Sphincter by Postoperative Vagus Nerve Injury: A Report on Two Cases
- Highly increased blood pressure following stellate ganglion block: A case report
- Anesthetic Experience of Vagus Nerve Stimulator Insertion for Intractable Epilepsy Patients: 18 Cases : A case report