Korean J Ophthalmol.
2014 Aug;28(4):306-313.
Intravitreal Anti-vascular Endothelial Growth Factor versus Observation in Acute Central Serous Chorioretinopathy: One-year Results
- Affiliations
-
- 1Department of Ophthalmology, Kangwon National University School of Medicine, Chuncheon, Korea. moosangkim@kangwon.ac.kr
Abstract
- PURPOSE
To evaluate the efficacy of anti-vascular endothelial growth factor (VEGF) compared with observation for treating acute central serous chorioretinopathy (CSC).
METHODS
A retrospective study of 36 patients with acute CSC, including 21 patients treated with anti-VEGF (anti-VEGF group) and 15 patients with observation (observation group). Patients in the anti-VEGF group received a single dose of bevacizumab or ranibizumab at baseline. Best-corrected visual acuity (BCVA), central foveal thickness (CFT) and resolution of subretinal fluid (SRF) on optical coherence tomography (OCT) were assessed. The integrity of the foveal inner segment/outer segment (IS/OS) line at 12 months was also analyzed.
RESULTS
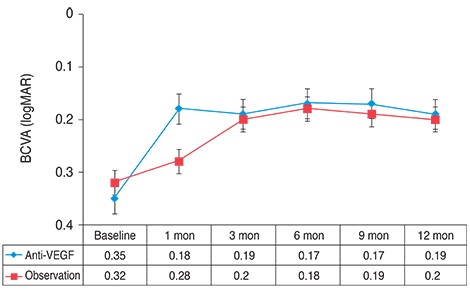
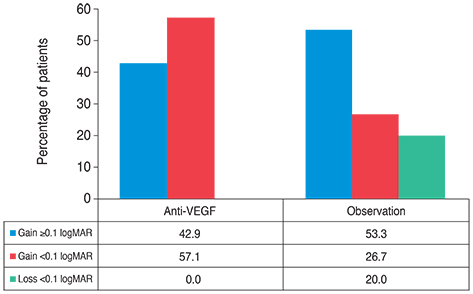
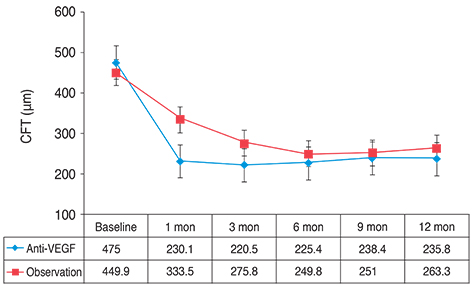
Resolution of SRF was achieved in 20 of 21 eyes in the anti-VEGF group and in 12 of 15 eyes in the observation group (p = 0.151). Mean BCVA and CFT were not different between the two groups at 12 months (p > 0.05). The amount of change in BCVA, however, differed significantly between the groups (p = 0.044). Final OCT more frequently detected the foveal IS/OS line in the anti-VEGF group than in the observation group (p = 0.012).
CONCLUSIONS
In terms of BCVA, anti-VEGF and observation only had similar therapeutic effects in acute CSC patients. In some patients, however, the rapid resolution of SRF by anti-VEGF might reduce the risk of photoreceptor degeneration and improve long-term visual acuity.
MeSH Terms
-
Acute Disease
Adult
Angiogenesis Inhibitors/*therapeutic use
Bevacizumab/therapeutic use
Central Serous Chorioretinopathy/*drug therapy/physiopathology
Female
Humans
Intravitreal Injections
Male
Middle Aged
Observation
Ranibizumab/therapeutic use
Retinal Photoreceptor Cell Inner Segment/pathology
Retinal Photoreceptor Cell Outer Segment/pathology
Retrospective Studies
Subretinal Fluid/drug effects
Tomography, Optical Coherence
Vascular Endothelial Growth Factor A/antagonists & inhibitors
Visual Acuity
Angiogenesis Inhibitors
Bevacizumab
Ranibizumab
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989; 96:854–859.2. Hussain D, Gass JD. Idiopathic central serous chorioretinopathy. Indian J Ophthalmol. 1998; 46:131–137.3. Loo RH, Scott IU, Flynn HW Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002; 22:19–24.4. Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol. 1983; 95:457–466.5. Taban M, Boyer DS, Thomas EL, Taban M. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol. 2004; 137:1073–1080.6. Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986; 9:37–41.7. Prunte C. Indocyanine green angiographic findings in central serous chorioretinopathy. Int Ophthalmol. 1995; 19:77–82.8. Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996; 121:26–34.9. Burumcek E, Mudun A, Karacorlu S, Arslan MO. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology. 1997; 104:616–622.10. Robertson DM. Argon laser photocoagulation treatment in central serous chorioretinopathy. Ophthalmology. 1986; 93:972–974.11. Cardillo Piccolino F, Eandi CM, Ventre L, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003; 23:752–763.12. Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003; 23:288–298.13. Chan WM, Lam DS, Lai TY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003; 87:1453–1458.14. Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina. 2006; 26:239–242.15. Stanga PE, Lim JI, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003; 110:15–21.16. Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol. 2008; 246:1235–1239.17. Lim JW, Kim MU. The efficacy of intravitreal bevacizumab for idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2011; 249:969–974.18. Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina. 1987; 7:111–131.19. Kanyange ML, De Laey JJ. Long-term follow-up of central serous chorioretinopathy (CSCR). Bull Soc Belge Ophtalmol. 2002; (284):39–44.20. Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994; 14:231–242.21. Lim JW, Kim MU, Shin MC. Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina. 2010; 30:1465–1471.22. Seong HK, Bae JH, Kim ES, et al. Intravitreal bevacizumab to treat acute central serous chorioretinopathy: short-term effect. Ophthalmologica. 2009; 223:343–347.23. Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010; 30:100–106.24. Lantry LE. Ranibizumab, a mAb against VEGF-A for the potential treatment of age-related macular degeneration and other ocular complications. Curr Opin Mol Ther. 2007; 9:592–602.25. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.26. Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010; 94:297–301.27. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011; 118:1594–1602.28. Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008; 19:208–212.29. Smretschnig E, Ansari-Shahrezaei S, Moussa S, et al. Half-fluence photodynamic therapy in acute central serous chorioretinopathy. Retina. 2012; 32:2014–2019.30. Zhao MW, Zhou P, Xiao HX, et al. Photodynamic therapy for acute central serous chorioretinopathy: the safe effective lowest dose of verteporfin. Retina. 2009; 29:1155–1161.31. Chan WM, Lai TY, Lai RY, et al. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008; 115:1756–1765.32. Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003; 121:695–706.33. Ko TH, Fujimoto JG, Duker JS, et al. Comparison of ultrahigh-and standard-resolution optical coherence tomography for imaging macular hole pathology and repair. Ophthalmology. 2004; 111:2033–2043.34. Matsumoto H, Kishi S, Otani T, Sato T. Elongation of photoreceptor outer segment in central serous chorioretinopathy. Am J Ophthalmol. 2008; 145:162–168.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Intravitreal Bevacizumab and Aflibercept Injections for Central Serous Chorioretinopathy
- Review and update for central serous chorioretinopathy
- The Effect of Intravitreal Bevacizumab in Patients with Acute Central Serous Chorioretinopathy
- Clinical Effects of Non-damaging Retinal Laser Therapy in Chronic Central Serous Chorioretinopathy
- Laser-Induced Choroidal Neovascularization in Central Serous Chorioretinopathy: 4 Cases