J Korean Med Sci.
2015 Sep;30(9):1328-1333. 10.3346/jkms.2015.30.9.1328.
Cognitive Dysfunction in Drug-induced Parkinsonism Caused by Prokinetics and Antiemetics
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, Hallym University College of Medicine, Anyang, Korea.
- 2Department of Neurology, Hallym University College of Medicine, Anyang, Korea. hima@hallym.ac.kr
- 3ILSONG Institute of Life Science, Hallym University College of Medicine, Anyang, Korea.
- 4Hallym Institute of Translational Genomics and Bioinformatics, Hallym University College of Medicine, Anyang, Korea.
- KMID: 2344160
- DOI: http://doi.org/10.3346/jkms.2015.30.9.1328
Abstract
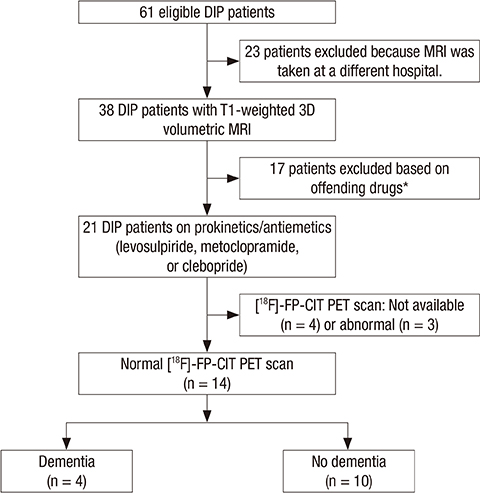
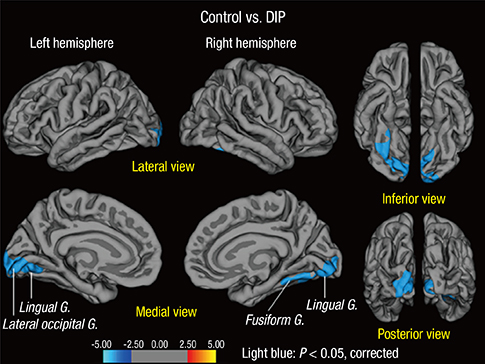
- The use of prokinetics/antiemetics is one of the leading causes of drug-induced parkinsonism (DIP) observed in neurology clinics. Cognitive dysfunction in DIP has recently been recognized, but pathologies related with cognitive dysfunction is unknown. Among our retrospective cohort of 385 consecutive parkinsonian patients enrolled in our parkinsonism registry, 14 patients were identified who satisfied our inclusion criteria: parkinsonism caused by prokinetics/antiemetics, existing T1-weighted 3D volumetric MR images, and normal [18F]-N-3-fluoropropyl-2-beta-carboxymethoxy-3-beta-(4-iodophenyl) nortropane PET scan images. For the comparison of volumetric MR data, 30 age- and sex-matched healthy individuals were included in this study. Among 14 patients with DIP, 4 patients were diagnosed with dementia, and all other patients had mild cognitive impairment (MCI). Comparisons of MR volumetric data between DIP patients with MCI and controls show that cortical gray matter volumes are reduced bilaterally in DIP (P=0.041) without changes in either total white matter volume or total intracranial volume. Among subcortical structures, the volume of the right hippocampus is reduced in DIP patients compared with controls (P=0.011, uncorrected). In DIP, cortical thickness is reduced in the bilateral lingual (P=0.002), right fusiform (P=0.032) and part of the left lateral occipital gyri (P=0.007). Our results suggests that cognitive dysfunction in DIP caused by prokinetics/antiemetics is common. Structural changes in the brain by 3D MRI may be associated with cognitive decline in DIP.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
Antiemetics/*adverse effects
Brain/drug effects/pathology
Cognition Disorders/*chemically induced/*pathology
Female
Gastrointestinal Agents/*adverse effects
Humans
Male
Parkinson Disease, Secondary/*chemically induced/*pathology
Republic of Korea
Retrospective Studies
Risk Assessment
Treatment Outcome
Antiemetics
Gastrointestinal Agents
Figure
Cited by 1 articles
-
Trends in the Prevalence of Drug-Induced Parkinsonism in Korea
Ji-Hye Byun, Hyemin Cho, Yun Joong Kim, Joong-Seok Kim, Jong Sam Baik, Sunmee Jang, Hyeo-Il Ma
Yonsei Med J. 2019;60(8):760-767. doi: 10.3349/ymj.2019.60.8.760.
Reference
-
1. Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology. 1999; 52:1214–1220.2. Sethi KD. Movement disorders induced by dopamine blocking agents. Semin Neurol. 2001; 21:59–68.3. Ma HI, Kim JH, Chu MK, Oh MS, Yu KH, Kim J, Hahm W, Kim YJ, Lee BC. Diabetes mellitus and drug-induced Parkinsonism: a case-control study. J Neurol Sci. 2009; 284:140–143.4. Shin HW, Kim MJ, Kim JS, Lee MC, Chung SJ. Levosulpiride-induced movement disorders. Mov Disord. 2009; 24:2249–2253.5. Kim JH, Byun HJ. Non-motor cognitive-perceptual dysfunction associated with drug-induced parkinsonism. Hum Psychopharmacol. 2009; 24:129–133.6. Kim JH, Kim SY, Byun HJ. Subjective cognitive dysfunction associated with drug-induced parkinsonism in schizophrenia. Parkinsonism Relat Disord. 2008; 14:239–242.7. López-Sendón J, Mena MA, de Yébenes JG. Drug-induced parkinsonism. Expert Opin Drug Saf. 2013; 12:487–496.8. Kim YD, Kim JS, Chung SW, Song IU, Yang DW, Hong YJ, Kim YI, Ahn KJ, Kim HT, Lee KS. Cognitive dysfunction in drug induced parkinsonism (DIP). Arch Gerontol Geriatr. 2011; 53:e222–e226.9. Shin HW, Kim JS, Oh M, You S, Kim YJ, Kim J, Kim MJ, Chung SJ. Clinical features of drug-induced parkinsonism based on [18F] FP-CIT positron emission tomography. Neurol Sci. 2015; 36:269–274.10. Kim JS, Oh YS, Kim YI, Yang DW, Chung YA, You Ie R, Lee KS. Combined use of (1)(2)(3)I-metaiodobenzylguanidine (MIBG) scintigraphy and dopamine transporter (DAT) positron emission tomography (PET) predicts prognosis in drug-induced Parkinsonism (DIP): a 2-year follow-up study. Arch Gerontol Geriatr. 2013; 56:124–128.11. Kim JH, Park J, Yu KH, Ma HI, Lee BC, Kim I, Kim YJ. Prodromal dementia with lewy bodies manifesting as sertraline-induced parkinsonism: a case report. Alzheimer Dis Assoc Disord. 2012; 26:191–193.12. Lee PH, Yeo SH, Yong SW, Kim YJ. Odour identification test and its relation to cardiac 123I-metaiodobenzylguanidine in patients with drug induced parkinsonism. J Neurol Neurosurg Psychiatry. 2007; 78:1250–1252.13. Kang Y, Na DL. Seoul Neuropsychological Screening Battery. 2nd ed. Incheon: Human Brain Research & Consulting Co.;2003.14. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999; 9:195–207.15. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002; 33:341–355.16. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000; 97:11050–11055.17. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999; 9:179–194.18. Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003; 20:2343–2356.19. Teipel SJ, Grothe M, Lista S, Toschi N, Garaci FG, Hampel H. Relevance of magnetic resonance imaging for early detection and diagnosis of Alzheimer disease. Med Clin North Am. 2013; 97:399–424.20. Seo SW, Im K, Lee JM, Kim YH, Kim ST, Kim SY, Yang DW, Kim SI, Cho YS, Na DL. Cortical thickness in single- versus multiple-domain amnestic mild cognitive impairment. Neuroimage. 2007; 36:289–297.21. Delli Pizzi S, Franciotti R, Tartaro A, Caulo M, Thomas A, Onofrj M, Bonanni L. Structural alteration of the dorsal visual network in DLB patients with visual hallucinations: a cortical thickness MRI study. PLoS One. 2014; 9:e86624.22. Pagonabarraga J, Corcuera-Solano I, Vives-Gilabert Y, Llebaria G, García-Sánchez C, Pascual-Sedano B, Delfino M, Kulisevsky J, Gómez-Ansón B. Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson’s disease. PLoS One. 2013; 8:e54980.