J Korean Ophthalmol Soc.
2014 Dec;55(12):1828-1833. 10.3341/jkos.2014.55.12.1828.
The Effect of Prophylactic IOP-Lowering Medication after Intravitreal Dexamethasone Implantation
- Affiliations
-
- 1Department of Ophthalmology, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Korea. syyu@khu.ac.kr
- KMID: 2338970
- DOI: http://doi.org/10.3341/jkos.2014.55.12.1828
Abstract
- PURPOSE
To investigate the effect of prophylactic intraocular pressure (IOP)-lowering medication after intravitreal dexamethasone implantation.
METHODS
This is a retrospective analysis of 39 eyes undergoing intravitreal dexamethasone implantation for macular edema. Eyes were divided into two groups, those which had used prophylactic IOP-lowering medication and those which had not. IOP was measured preoperatively, at one week, and monthly until six months post-injection in each group.
RESULTS
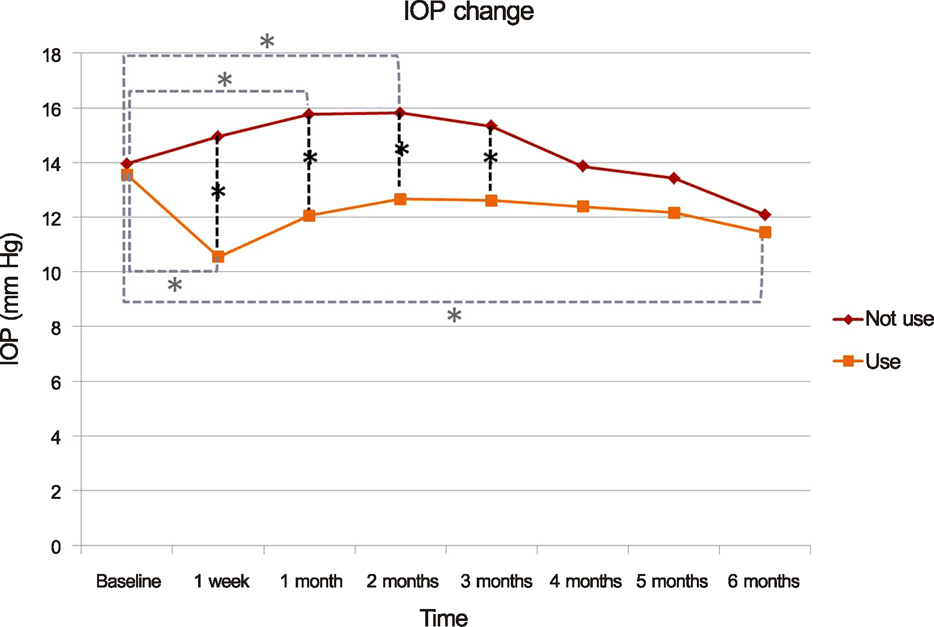
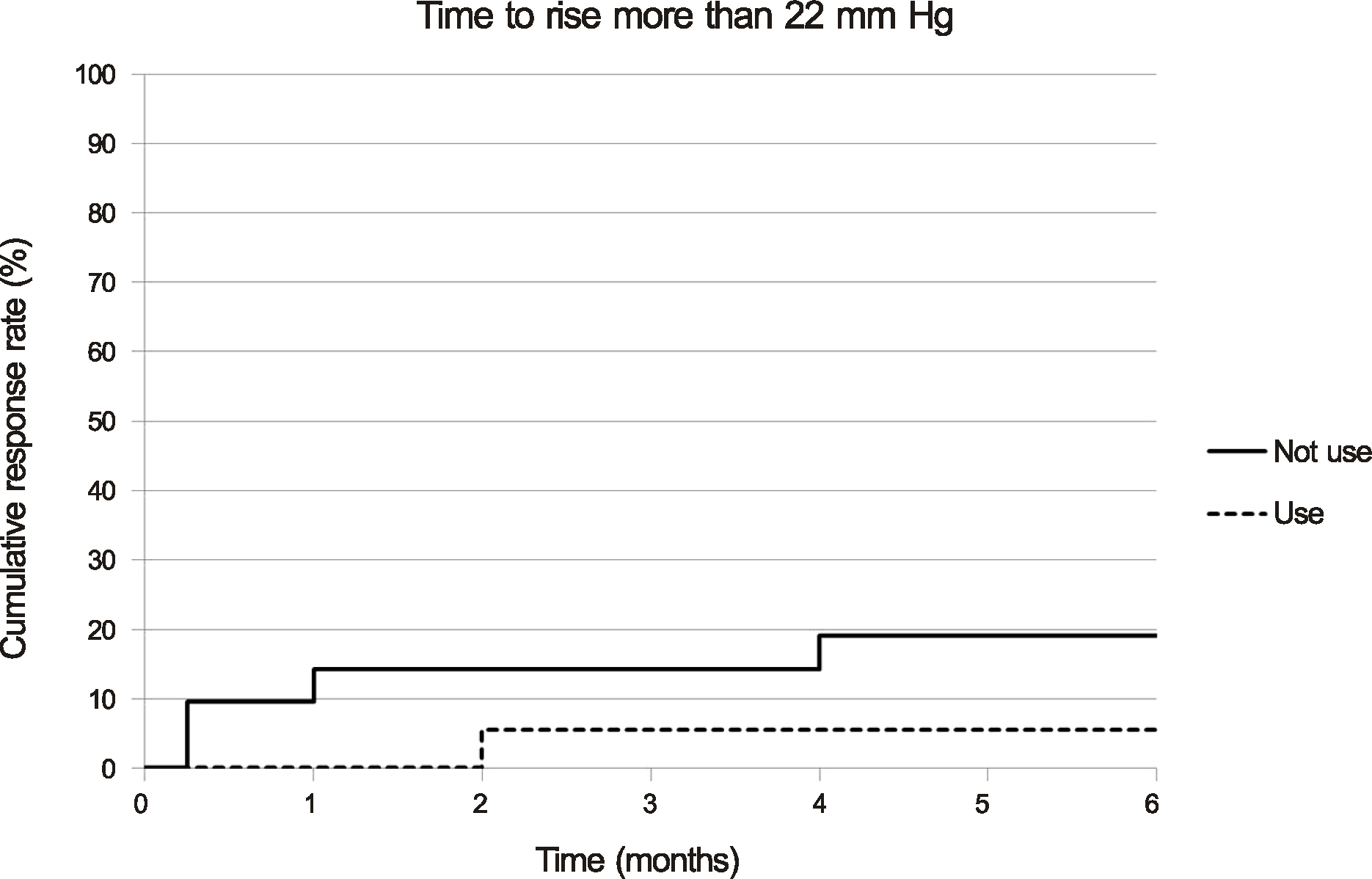
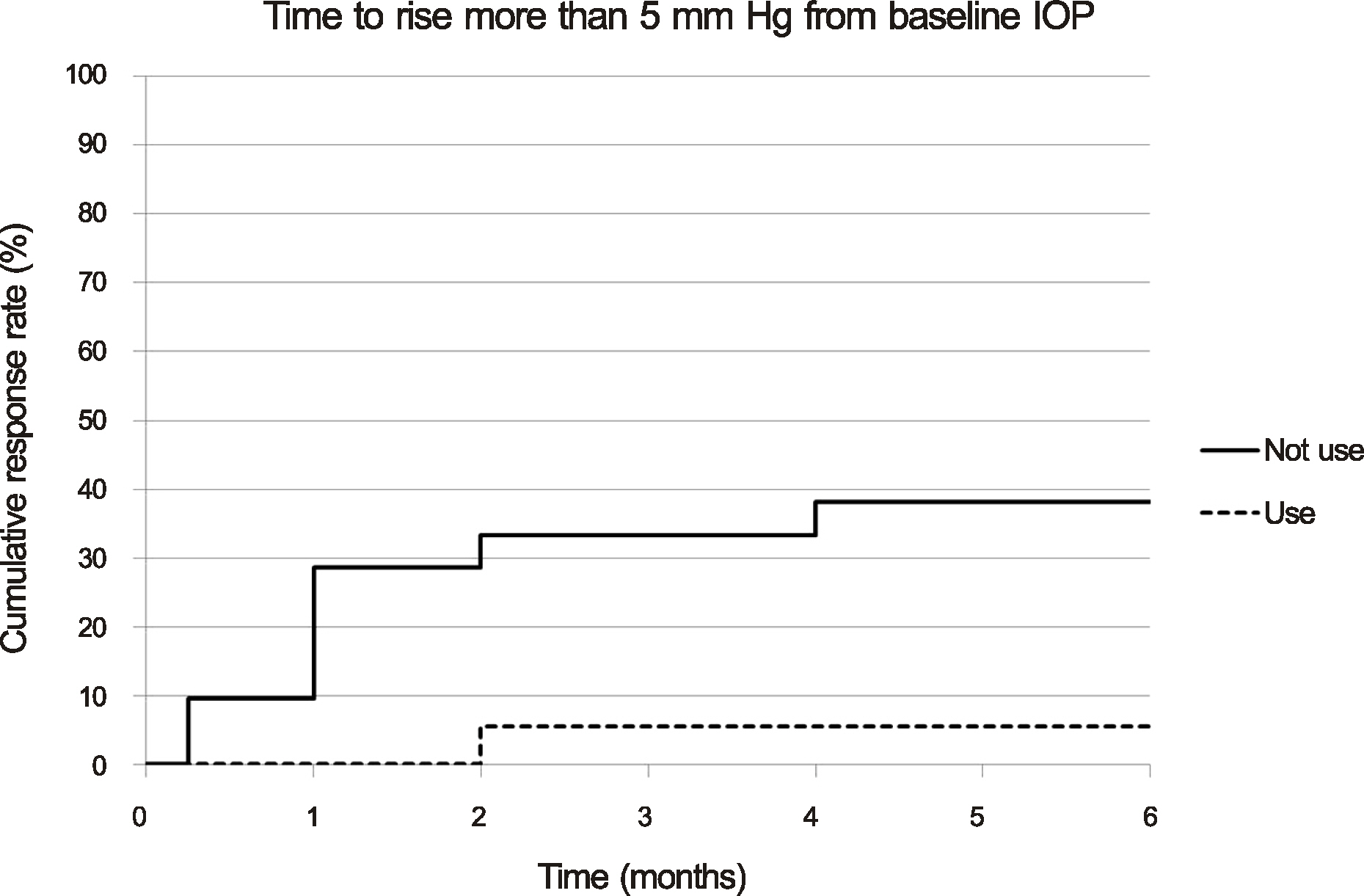
The mean pre-injection IOP for the group that had not used prophylactic IOP-lowering medication and the group that had was 13.95 +/- 3.32 mm Hg and 13.56 +/- 3.71 mm Hg, the mean post-injection IOP at two months was 15.81 +/- 3.75 mm Hg and 12.56 +/- 5.02 mm Hg, and that at six months was 12.90 +/- 2.95 mm Hg and 11.44 +/- 3.59 mm Hg, respectively. The difference between the two groups was statistically significant at one week, one month, two months, and three months (p = 0.001, 0.002, 0.011, 0.035, respectively). A greater than 22 mm Hg increase in IOP was seen in four eyes (19.05%) in the group that had not used IOP-lowering medication and in one eye (5.56%) in the group that had. A greater than 5 mm Hg increase in IOP from baseline was seen in eight eyes (38.10%) in the group that had not used IOP-lowering medication and in one eye (5.56%) in the group that had.
CONCLUSIONS
After intravitreal dexamethasone implantation, prophylactic IOP-lowering medication will significantly prevent IOP increase and decrease the number of patients requiring additional treatment that could cause potential damage to the retina and optic nerve.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Intraocular Pressure Changes in Patients with Retinal Vein Occlusion Treated with Intravitreal Dexamethasone Implant Insertion
Juno Kim, Sung Jin Lee, Dong Hyun Wang
J Korean Ophthalmol Soc. 2018;59(6):519-526. doi: 10.3341/jkos.2018.59.6.519.
Reference
-
References
1. Ferris FL 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984; 28(Suppl):452–61.
Article2. Durrani OM, Tehrani NN, Marr JE, et al. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004; 88:1159–62.
Article3. Orth DH, Patz A. Retinal branch vein occlusion. Surv Ophthalmol. 1978; 22:357–76.
Article4. Kiernan DF, Mieler WF. The use of intraocular corticosteroids. Expert Opin Pharmacother. 2009; 10:2511–25.
Article5. Mansoor S, Kuppermann BD, Kenney MC. Intraocular sustained-release delivery systems for triamcinolone acetonide. Pharm Res. 2009; 26:770–84.
Article6. Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008; 16:791–9.
Article7. Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002; 133:70–7.
Article8. Patel JI, Tombran-Tink J, Hykin PG, et al. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: Implications for structural differences in macular profiles. Exp Eye Res. 2006; 82:798–806.
Article9. Haller JA, Band, ello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.e3.
Article10. Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011; 31:915–23.
Article11. Meyer LM, Schönfeld CL. Secondary glaucoma after intravitreal dexamethasone 0.7 mg implant in patients with retinal vein occlusion: a one-year follow-up. J Ocul Pharmacol Ther. 2013; 29:560–5.12. Lewis JM, Priddy T, Judd J, et al. Intraocular pressure response to topical dexamethasone as a predictor for the development of primary open-angle glaucoma. Am J Ophthalmol. 1988; 106:607–12.
Article13. Jampol LM, Yannuzzi LA, Weinreb RN. Glaucoma and intravitreal steroids. Ophthalmology. 2005; 112:1325–6.
Article14. Wilson K, McCartney MD, Miggans ST, Clark AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Curr Eye Res. 1993; 12:783–93.
Article15. Clark AF, Wilson K, McCartney MD, et al. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994; 35:281–94.16. Johnson DH, Bradley JM, Acott TS. The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Invest Ophthalmol Vis Sci. 1990; 31:2568–71.17. Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999; 18:629–67.
Article18. Snyder RW, Stamer WD, Kramer TR, Seftor RE. Corticosteroid treatment and trabecular meshwork proteases in cell and organ culture supernatants. Exp Eye Res. 1993; 57:461–8.
Article19. Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006; 17:163–7.20. Bozkurt E, Kara N, Yazici AT, et al. Prophylactic selective laser trabeculoplasty in the prevention of intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2011; 152:976–81.e2.
Article21. Lee MW, Kyung SE, Chang MH. Prophylactic effect of brimonidine 0.15% on IOP elevation after intravitreal triamcinolone acetonide injection. J Korean Ophthalmol Soc. 2008; 49:743–52.
Article22. Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011; 52:80–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intraocular Pressure Elevation after 0.7 mg Intravitreal Dexamethasone (Ozurdex(R)) Implantation: A One Year Follow-Up
- A Case of Repeated Intravitreal Dexamethasone Implantation for Treatment of Macular Edema after Scleral Fixation of Intraocular Lens
- Intraocular Pressure Changes in Patients with Retinal Vein Occlusion Treated with Intravitreal Dexamethasone Implant Insertion
- Risk Factors and Incidence of Elevated Intraocular Pressure after Dexamethasone Intravitreal Implant
- Prophylactic Effect of Brimonidine 0.15% on IOP Elevation After Intravitreal Triamcinolone Acetonide Injection