J Korean Ophthalmol Soc.
2016 Sep;57(9):1422-1429. 10.3341/jkos.2016.57.9.1422.
Risk Factors and Incidence of Elevated Intraocular Pressure after Dexamethasone Intravitreal Implant
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University School of Medicine, Busan, Korea. glaucoma@pnu.ac.kr
- 2Department of Ophthalmology, Maryknoll Medical Center, Busan, Korea.
- 3Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 2351873
- DOI: http://doi.org/10.3341/jkos.2016.57.9.1422
Abstract
- PURPOSE
To report the incidence of intraocular pressure (IOP) elevation and identify the risk factors of IOP elevation after intravitreal dexamethasone 0.7 mg (Ozurdex®, Allergan, Irvine, CA, USA) implant.
METHODS
A total of 86 eyes of 79 patients who underwent intravitreal dexamethasone implantation and who were followed for ≥ 3 months were included in the present study. IOP elevation was defined as a pressure > 21 mm Hg at some time during follow-up.
RESULTS
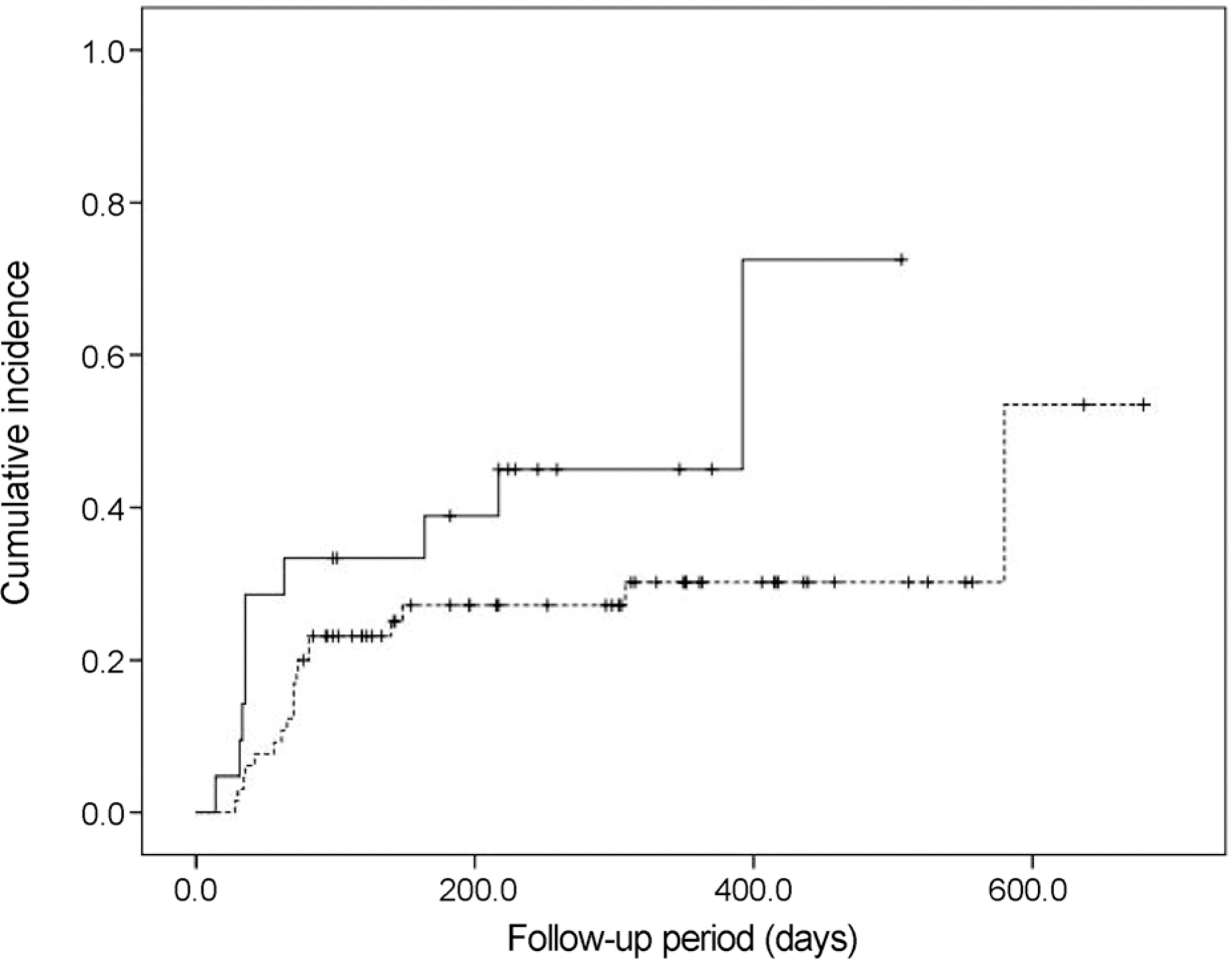
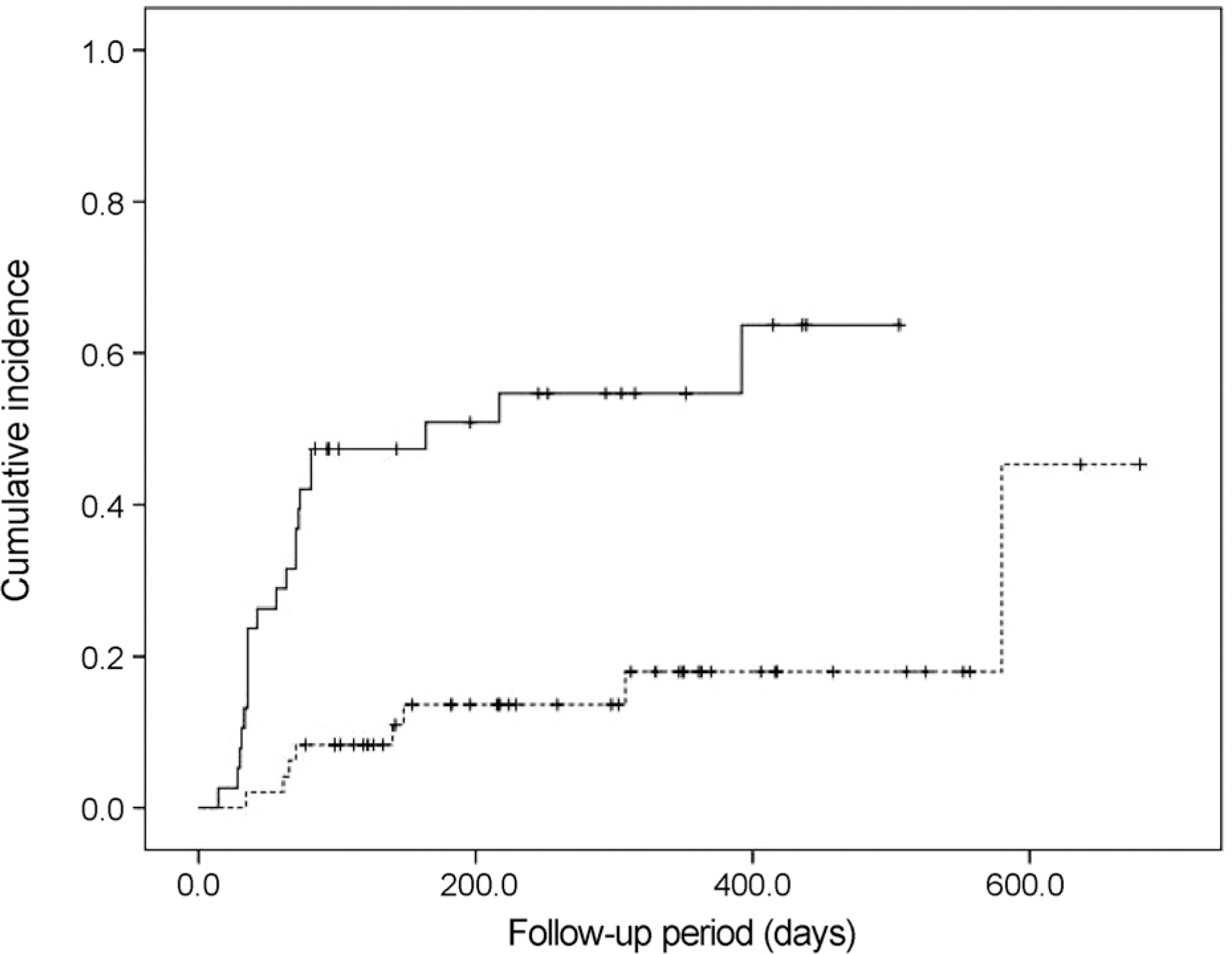
Twenty-nine eyes (33.7%) had an IOP > 21 mm Hg after dexamethasone intravitreal implant. The incidence of IOP elevation increased rapidly at 2-3 months after dexamethasone intravitreal implant. The Kaplan-Meier estimated incidence of IOP elevation was 25.6 ± 4.7% (mean ± standard error) at 81 days. Cox multivariate analysis showed the significant risk factors of IOP elevation to be age < 55 years (p = 0.045), baseline IOP ≥ 15 mm Hg (p < 0.001), and history of intraocular surgery (p = 0.039).
CONCLUSIONS
This study demonstrates the incidence of IOP elevation to be 33.7% and describes the risk factors associated with IOP elevation. Clinicians should be cautious regarding the possibility of IOP elevation after intravitreal dexamethasone implant, especially in the presence of identified risk factors.
MeSH Terms
Figure
Reference
-
References
1. Chan CK, Fan DS, Chan WM, et al. Ocular-hypertensive response and corneal endothelial changes after intravitreal triamcinolone abdominals in Chinese subjects: a 6-month follow-up study. Eye (Lond). 2005; 19:625–30.2. Bernstein HN, Schwartz B. Effects of long-term systemic steroids on ocular pressure and tonographic values. Arch Ophthalmol. 1962; 68:742–53.
Article3. Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992; 10:505–10.
Article4. Jonas J, Heatley G, Spaide R, Varma R. Intravitreal triamcinolone acetonide and secondary ocular hypertension. J Glaucoma. 2005; 14:168–71.
Article5. Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal abdominal acetonide and intraocular pressure. Am J Ophthalmol. 2004; 138:740–3.6. Yang YH, Kim KR, Yang SW, Yim HB. The effect of intravitreal triamcinolone acetonide on intraocular pressure. J Korean Ophthalmol Soc. 2004; 45:1081–5.7. Ozkiriş A, Erkiliç K. Complications of intravitreal injection of abdominal acetonide. Can J Ophthalmol. 2005; 40:63–8.8. Park HY, Yi K, Kim HK. Intraocular pressure elevation after abdominal triamcinolone acetonide injection. Korean J Ophthalmol. 2005; 19:122–7.9. Mazzarella S, Mateo C, Freixes S, et al. Effect of intravitreal abdominal of dexamethasone 0.7 mg (Ozurdex(R)) on intraocular pressure in patients with macular edema. Ophthalmic Res. 2015; 54:143–9.10. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-abdominalled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.e3.11. Ryder SJ, Iannetta D, Bhaleeya SD, Kiss S. Efficacy and abdominal of bilateral sustained-release dexamethasone intravitreal implants for the treatment of noninfectious posterior uveitis and macular edema secondary to retinal vein occlusion. Clin Ophthalmol. 2015; 9:1109–16.12. Park DH, Ha SJ, Lee SJ. Intraocular pressure elevation after 0.7 mg intravitreal dexamethasone (Ozurdex(R)) implantation: a one year follow-up. J Korean Ophthalmol Soc. 2015; 56:891–9.13. Roth DB, Verma V, Realini T, et al. abdominal incidence and timing of intraocular hypertension after intravitreal triamcinolone acetonide injection. Ophthalmology. 2009; 116:455–60.14. Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effects of dexamethasone in the abdominal eye. Arch Ophthalmol. 1963; 70:492–9.15. Becker B, Hahn KA. Topical corticosteroids and heredity in abdominal open-angle glaucoma. Am J Ophthalmol. 1964; 57:543–51.16. Podos SM, Becker B, Morton WR. High myopia and primary open-angle glaucoma. Am J Ophthalmol. 1966; 62:1038–43.
Article17. Becker B. Diabetes mellitus and primary open-angle glaucoma. The XXVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 1971; 71(1 Pt 1):1–16.18. Gaston H, Absolon MJ, Thurtle OA, Sattar MA. Steroid responsiveness in connective tissue diseases. Br J Ophthalmol. 1983; 67:487–90.
Article19. Spaeth GL. Traumatic hyphema, angle recession, dexamethasone hypertension, and glaucoma. Arch Ophthalmol. 1967; 78:714–21.
Article20. Becker B, Podos SM. Krukenberg's spindles and primary open-abdominal glaucoma. Arch Ophthalmol. 1966; 76:635–9.21. Haas JS, Nootens RH. Glaucoma secondary to benign adrenal adenoma. Am J Ophthalmol. 1974; 78:497–500.
Article22. Lau LI, Chen KC, Lee FL, et al. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection in a Chinese population. Am J Ophthalmol. 2008; 146:573–8.
Article23. Callanan DG, Gupta S, Boyer DS, et al. Dexamethasone abdominal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013; 120:1843–51.24. Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in abdominals with diabetic macular edema. Ophthalmology. 2014; 121:1904–14.25. Bakri SJ, Omar AF, Lezzi R, Kapoor KG. Evaluation of multiple dexamethasone intravitreal implants in patients with macular abdominal associated with retinal vein occlusion. Retina. 2016; 36:552–7.26. Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014; 121:2473–81.27. Leung YF, Tam PO, Lee WS, et al. The dual role of dexamethasone on anti-inflammation and outflow resistance demonstrated in abdominal human trabecular meshwork cells. Mol Vis. 2003; 9:425–39.28. Rozsa FW, Reed DM, Scott KM, et al. Gene expression profile of human trabecular meshwork cells in response to long-term abdominal exposure. Mol Vis. 2006; 12:125–41.29. Nehmé A, Lobenhofer EK, Stamer WD, Edelman JL. Glucocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med Genomics. 2009; 2:58.
Article30. Rhee DJ, Peck RE, Belmont J, et al. Intraocular pressure alterations following intravitreal triamcinolone acetonide. Br J Ophthalmol. 2006; 90:999–1003.
Article31. Knepper PA, Breen M, Weinstein HG, Blacik JL. Intraocular abdominal and glycosaminoglycan distribution in the rabbit eye: effect of age and dexamethasone. Exp Eye Res. 1978; 27:567–75.32. Wordinger RJ, Clark AF. Effects of glucocorticoids on the abdominal meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999; 18:629–67.33. Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006; 17:163–7.34. Alexander JP, Samples JR, Van Buskirk EM, Acott TS. Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork. Invest Ophthalmol Vis Sci. 1991; 32:172–80.35. Yildirim N, Sahin A, Erol N, et al. The relationship between abdominal MMP-9 and TIMP-2 levels and intraocular pressure elevation in diabetic patients after intravitreal triamcinolone injection. J Glaucoma. 2008; 17:253–6.36. Laplace O, Rodallec T, Akesbi J, Sandali O. Anterior chamber migration of a dexamethasone implant in a pseudophakic patient with a scleral-fixated posterior chamber intraocular lens. J Fr Ophtalmol. 2013; 36:e59–61.37. Schmidt JC, Meyer CH, Mennel S. Parsplana vitrectomy with abdominal chamber infusion via a paracentesis in pseudophakic eyes. Ophthalmologe. 2007; 104:222–5.38. Yanyali A, Aytug B, Horozoglu F, Nohutcu AF. Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am J Ophthalmol. 2007; 144:124–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intraocular Pressure Elevation after 0.7 mg Intravitreal Dexamethasone (Ozurdex(R)) Implantation: A One Year Follow-Up
- Intraocular Pressure Changes in Patients with Retinal Vein Occlusion Treated with Intravitreal Dexamethasone Implant Insertion
- A Case of Repeated Intravitreal Dexamethasone Implantation for Treatment of Macular Edema after Scleral Fixation of Intraocular Lens
- Therapeutic Efficacy of Intravitreal Dexamethasone Implant in Korean Patients with Non-infectious Uveitis
- Intravitreal Injection of Dexamethasone Implant during Cataract Surgery in Patients with Noninfectious Uveitis