J Korean Ophthalmol Soc.
2012 Feb;53(2):342-347.
Moxifloxacin Mixed Augmented Amniotic Membrane Transplantation for Perforating Infectious Keratitis
- Affiliations
-
- 1Department of Ophthalmology, Kyungpook National University School of Medicine, Daegu, Korea. okeye@knu.ac.kr
Abstract
- PURPOSE
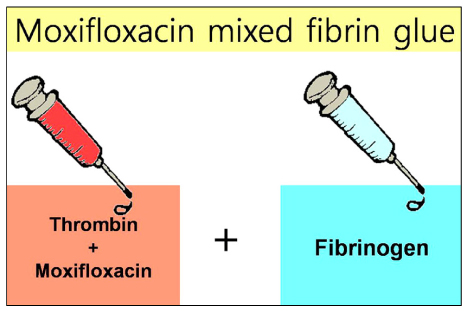
To report the clinical results of moxifloxacin mixed augmented amniotic membrane transplantation (AMT) in 2 patients with perforating infectious keratitis.
CASE SUMMARY
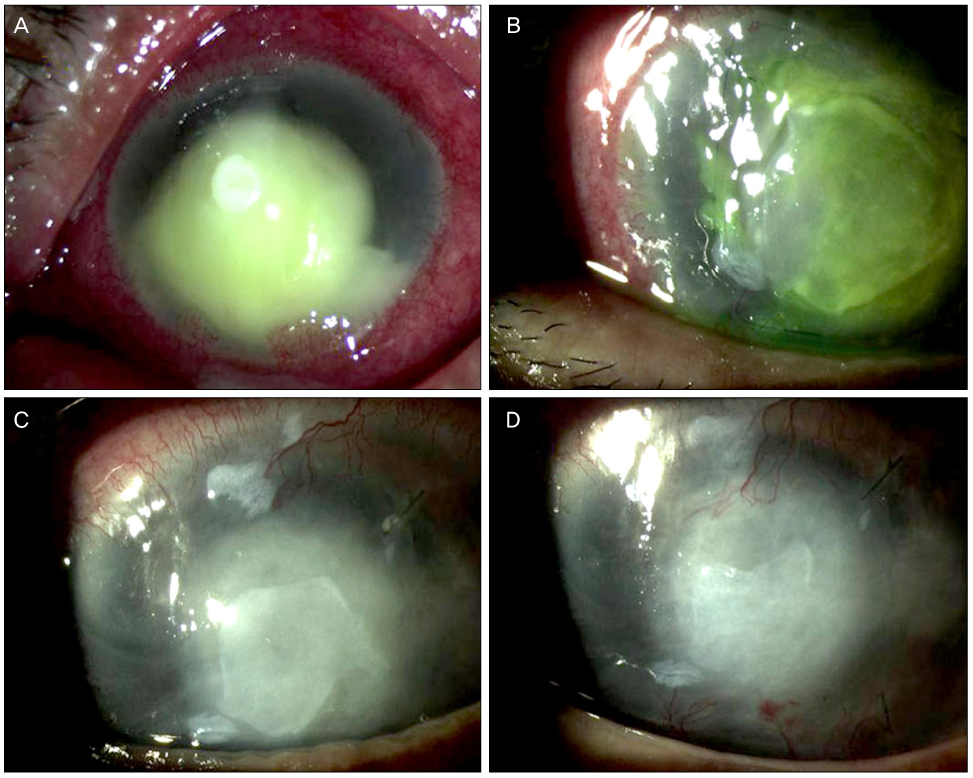
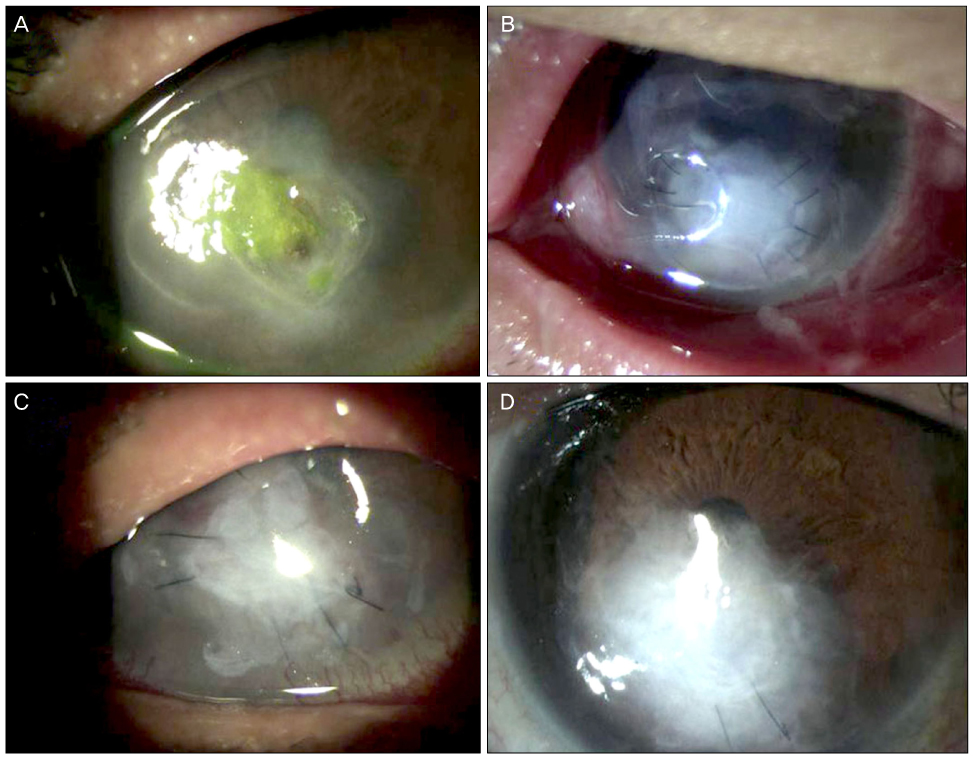
Moxifloxacin mixed augmented amniotic membrane transplantations were performed in 2 patients with rapidly deteriorating deep perforated bacterial keratitis. All patients preserved their eyesight. Complete re-epithelization over the amniotic membrane were observed within a month. The corneal surfaces were healed with opacity, and there were no active infectious infiltrations or recurrences for 3 months after application.
CONCLUSIONS
Moxifloxacin mixed augmented AMT has proven to be successful both tectonically and physiologically for cases with perforating active bacterial keratitis.
MeSH Terms
Figure
Reference
-
1. Bessant DA, Dart JK. Lamellar keratoplasty in the management of inflammatory corneal ulceration and perforation. Eye. 1994. 8:22–28.2. Nobe JR, Moura BT, Robin JB, et al. Results of penetrating keratoplasty for the treatment of corneal perforations. Arch Ophthalmol. 1990. 108:939–941.3. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995. 14:473–484.4. Kruse FE, Rohrschneider K, Völcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999. 106:1504–1510.5. Oh JH, Kim YY, Song JS. Corneal perforation caused by noninfectious corneal ulcer in a patient with toxic epidermal necrolysis. J Korean Ophthalmol Soc. 2006. 47:1829–1833.6. Kim HK, Park HS. Fibrin glue-assisted augmented amniotic membrane transplantation for the treatment of large noninfectious corneal perforations. Cornea. 2009. 28:170–176.7. Xu JJ, Wang Y. The effects of amniotic membrane on corneal penetration of ofloxacin. Zhonghua Yan Ke Za Zhi. 2006. 42:624–627.8. Lee SY, Heo JW, Wee WR, et al. A case of endophthalmitis with necrotizing scleritis treated with vitrectomy and permanent amniotic membrane transplantation. J Korean Ophthalmol Soc. 2011. 52:97–102.9. Hick S, Demers PE, Brunette I, et al. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases. Cornea. 2005. 24:369–377.10. Rodríguez-Ares MT, Touriño R, López-Valladares MJ, Gude F. Multilayer amniotic membrane transplantation in the treatment of corneal perforations. Cornea. 2004. 23:577–583.11. Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 2004. 88:911–914.12. Duchesne B, Tahi H, Galand A. Use of human fibrin glue and amniotic membrane transplant in corneal perforation. Cornea. 2001. 20:230–232.13. Kim JS, Kim JC, Na BK, et al. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000. 70:329–337.14. Wang MX, Gray TB, Park WC, et al. Reduction in corneal haze and apoptosis by amniotic membrane atrix in excimer laser photoablation in rabbits. J Cataract Refract Surg. 2001. 27:310–319.15. Lambiase A, Sacchetti M, Sgrulletta R, et al. Amniotic membrane transplantation associated with conjunctival peritomy in the management of Mooren's ulcer: a case report. Eur J Ophthalmol. 2005. 15:274–276.16. Kjaergaard N, Hein M, Hyttel L, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001. 94:224–229.17. Kjaergaard N, Helmig RB, Schønheyder HC, et al. Chorioamniotic membranes constitute a competent barrier to group B Streptococcus in vitro. Eur J Obstet Gynecol Reprod Biol. 1999. 83:165–169.18. Kim HS, Sah WJ, Kim YJ, et al. Amniotic membrane, tear film, corneal, and aqueous levels of ofloxacin in rabbit eyes after amniotic membrane transplantation. Cornea. 2001. 20:628–634.19. Kowalski RP, Romanowski EG, Mah FS, et al. Intracameral Vigamox (moxifloxacin 0.5%) is non-toxic and effective in preventing endophthalmitis in a rabbit model. Am J Ophthalmol. 2005. 140:497–504.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cases of Amniotic Membrane Transplantation for Herpetic Keratitis
- Therapeutic Effect of Amniotic Membrane Transplantation in Active Bacterial Corneal Ulcer
- A Case of Mycobacterium Tuberculosis Keratitis
- Human Amniotic Membrane Transplantation for Treatment of Fungal Ulcer
- Ocular Surface Reconstruction with Amniotic Membrane Transplantation in Pterygium