J Korean Soc Clin Pharmacol Ther.
2012 Dec;20(2):155-164.
Statistical Analysis System of Spontaneous Adverse Drug Reaction Reports
- Affiliations
-
- 1Graduate School of Healthcare Management and Policy, The Catholic of the University, Catholic Medical Center, Seoul, Korea. iychoi@catholic.ac.kr
- 2Seoul St' Mary Hospital, The Catholic of the University, Catholic Medical Center, Seoul, Korea.
- 3Department of Pharmacology, The Catholic of the University, Catholic Medical Center, Seoul, Korea.
- 4Department of Dermatology, The Catholic of the University, Catholic Medical Center, Seoul, Korea.
Abstract
- BACKGROUND
Spontaneous adverse drug reaction (ADR) reporting data has been used for safety of post-market drug surveillance. A system has been required that is able to detect signals associated with drugs by analyzing the collected ADR data.
METHODS
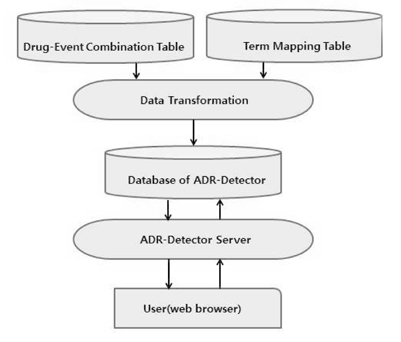
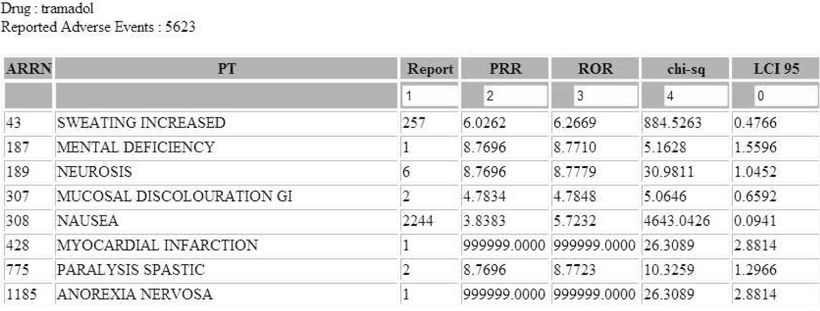
We developed the web-based automated analysis system (ADR-detector). We used the data which reported ADR spontaneously between March 2009 and December 2010 to Korean Food and Drug Administration. We used 3 statistical indicators for evaluating ADR signals: proportional reporting ratio (PRR), reporting odds ratio (ROR), and information component (IC). The ADR reports which were detected as significant signals based on the indicators have been reviewed.
RESULTS
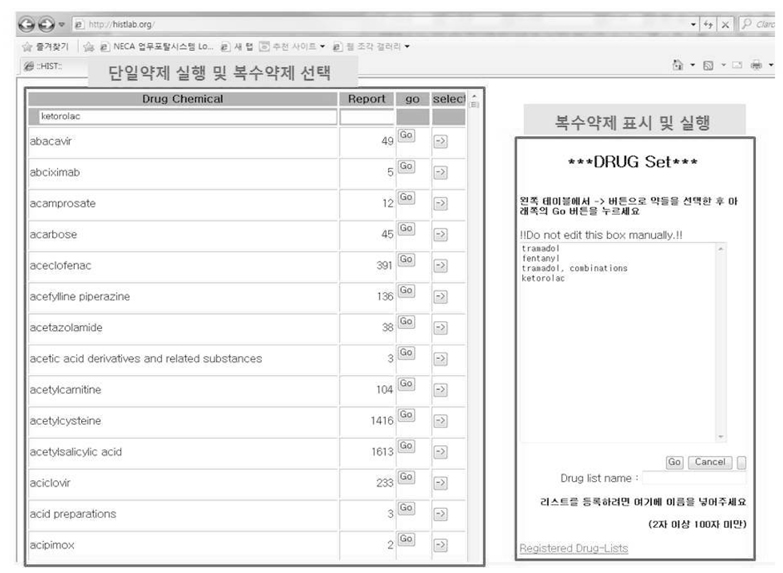
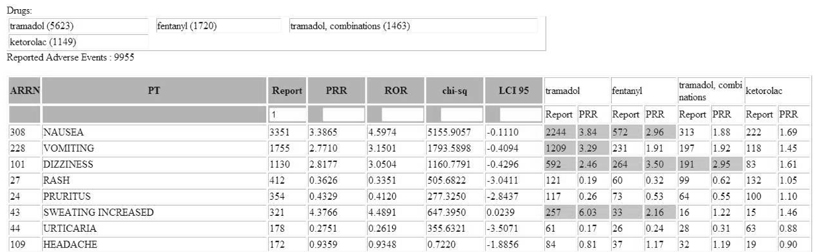
Among 153,774 reports, 9,955 cases were related to 4 analgesics which were most frequently reported analgesic drugs during the study period. The numbers of ADR reports associated with each drug are as follow: 5,623 reports in tramadol (56.5 %), 1,720 reports in fentanyl (17.3 %), 1,463 reports in tramadol-combination (14.7 %), and 1,149 reports in ketorolac (11.5 %). Top 5 ADR were nausea (3,351 reports - 33.7 %), vomiting (1,755 reports - 17.6 %), dizziness (1,130 - 11.4 %), rash (412 reports - 4.1 %), and pruritus (354 reports - 3.6 %). 6,674 ADR reports were significant based on PRR and ROR, and 336 reports were significant based on IC.
CONCLUSION
By using the automated analysis system, not only statisticians but also general researchers are able to analyze ADR signals in real-time. Also ADR-detector would provide rapid review and cross-check of ADR.
MeSH Terms
Figure
Reference
-
1. Park BJ. Development of statistical methods for analyzing drug adverse event data. Annu Rep Korea Food Drug Adm. 2007. 7–14.2. Park S, Chae SM. Social Relief Scheme for Serious Adverse Drug Reactions-Lessons from other countries for Korea. J Korean Soc Clin Pharmacol Ther. 2008. 18(1):18–27. (Korean).3. Fletcher AP. Spontaneous adverse drug reaction reporting vs event monitoring: a comparison. J R Soc Med. 1991. 84(6):341–344.
Article4. 2007 Statistical Annual Report of Food & Drug. 2007. (9):Korea Food & Drug Administration;205–207.5. Kim YS. A study on the plan for activating adverse drug reaction monitoring. Annu Rep Korea Food Drug Adm. 2008. 12:611–612.6. Seong JM, Choi NK, Jung SY, Kim YJ, Lee JY, Park BJ. Signal Detection of Sildenafil in Korean Spontaneous Adverse Event Reports. J Pharmacoepidemiol Risk Manag. 2009. 2(1):38–44. (Korean).7. Van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002. 11(1):3–10.
Article8. Song YJ, Ha CW. The Use of COX-2 Selective Nonsteroidal Anti-inflammatory Drugs for the Treatment of Osteoarthritis. J Korean Knee Soc. 2009. 21(2):84–92. (Korean).9. Ha JH. Risk signal detection of marketed NSNNAs (non-steroidal and non-narcotic analgesics). 2011. Doctoral Dissertation Sungkyun Kwan University. (Korean).10. Kim MG, Kang HR, Kim JH, Ju YS, Park SH, Hwang YI, Jang SH, Kim DG, Jung KS. Analysis of adverse drug reactions collected by an electronic reporting system in a single hospital. Korean J Med. 2009. 77(5):601–609. (Korean).11. Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001. 10(6):483–486.
Article12. Egberts AC, Meyboom RH, van Puijenbroek EP. Use of meaures of disproportionality in pharmacovigilance: three Dutch examples. Drug Saf. 2002. 25(6):453–458.13. Lindquist M, Ståhl M, Bate A, Edwards IR, Meyboom RH. A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf. 2000. 23(6):533–542.
Article14. Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK. Adverse drug reactions. BMJ. 1998. 316:1295–1298.15. Einarson TR. Drug-related hospital admissions. Ann Pharmacother. 1993. 27(7-8):832–840.
Article16. Morimoto T, Gandhi TK, Segar AC, Hsieh TC, Bates DW. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004. 13:306–314.
Article17. Kopečná E, Deščíková V, Vlček J, Mladá J. Adverse drug reaction reporting in the Czech Republic 2005-2009. Int J Clin Pharm. 2011. 33(4):683–689.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fentanyl PCA Monotherapy and Fentanyl TTS Combination Therapy in Post-Operative Pain Management: Analyses of Spontaneous Adverse Drug Reaction Reports
- Signal Detection of Rosuvastatin Calcium Using Spontaneous Adverse Drug Event Reports
- Signal Detection and Safety Information Generation of Aripiprazole in Spontaneous Adverse Event Reports Database
- Adverse Drug Reaction Surveillance System in Korea
- Pediatric Medication Error Reports in Korea Adverse Event Reporting System Database, 1989-2012: Comparing with Adult Reports