J Periodontal Implant Sci.
2010 Aug;40(4):164-171.
Antioxidant profile of whole saliva after scaling and root planing in periodontal disease
- Affiliations
-
- 1Department of Periodontology, Chonnam National University School of Dentistry, Gwangju, Korea. periodrk@chonnam.ac.kr
- 2Dental Research Institute, Chonnam National University School of Dentistry, Gwangju, Korea.
- 3Department of Oral Pathology, Chonnam National University School of Dentistry, Gwangju, Korea.
Abstract
- PURPOSE
This study compared the total antioxidant status (TAS) and superoxide dismutase (SOD) activity in the saliva of periodontally compromised patients before and after scaling and root planing (SRP) to assess their diagnostic utility.
METHODS
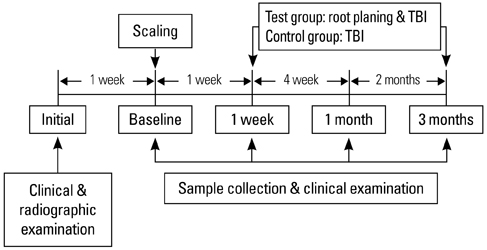
Severe chronic periodontitis patient (test group) and subjects with no attachment loss, sites showing a 3 mm or more probing depth and a sulcus bleeding index < 10% (control group) were enrolled in this study. Saliva sampling and clinical examination were performed at one week, one month and 3 months after SRP. The TAS and SOD activity in each patient's saliva was measured for the comparative analysis between the groups.
RESULTS
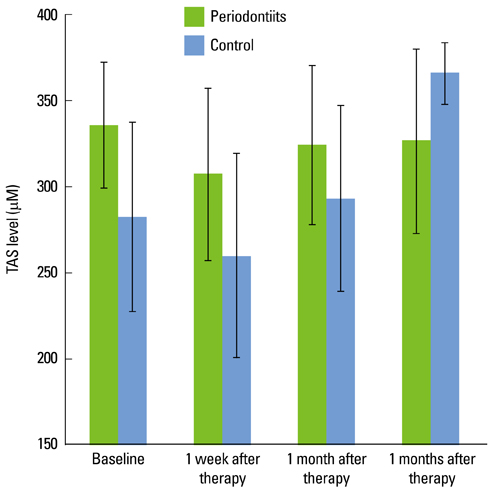
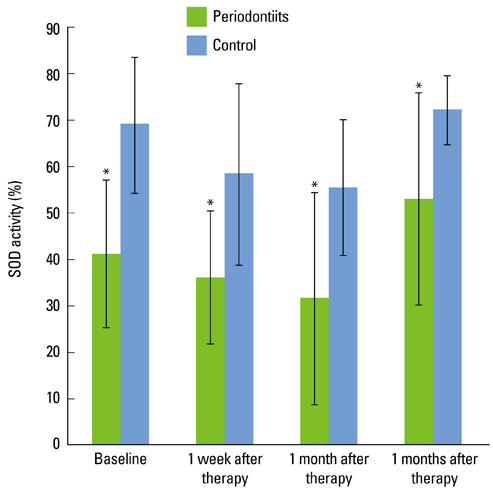
In the test group, the TAS decreased directly after SRP. With time, it increased slightly and was relatively unchanged compared to the baseline. In the control group, the TAS also decreased immediately after SRP but increased gradually with time until 3 months. The SOD activity in the test and control subjects decreased immediately after SRP until 1 month. At 3 months, the SOD activity had increased. Both groups had a similar profile of SOD activity. However, the SOD activity of the control group was significantly higher than that of the test group at each point in time (P < 0.05).
CONCLUSIONS
There was a significant difference in the total salivary antioxidant level between the periodontitis and healthy or gingivitis (control) group during the experiment period. The total antioxidant level in the saliva was higher in the patients with severe chronic periodontitis than the healthy or gingivitis control before SRP. The SOD activity of the periodontitis patients was lower than the control at each time point. These findings conclusively reveal the possible use of saliva as a diagnostic tool for periodontal health.
Keyword
MeSH Terms
Figure
Reference
-
1. Forrest JL, Hujoel PP, Lieberman MB. Newman MG, Takei HH, Carranza FA, editors. Host modulation. Carranza's clinical periodontology. 2006. 10th ed. St. Louis: Saunders/Elsevier;275–282.2. Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997. 24:287–296.
Article3. Bartold PM, Wiebkin OW, Thonard JC. The effect of oxygen-derived free radicals on gingival proteoglycans and hyaluronic acid. J Periodontal Res. 1984. 19:390–400.
Article4. Varani J, Fligiel SE, Till GO, Kunkel RG, Ryan US, Ward PA. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985. 53:656–663.5. Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991. 91:14S–22S.
Article6. Akalin FA, Toklu E, Renda N. Analysis of superoxide dismutase activity levels in gingiva and gingival crevicular fluid in patients with chronic periodontitis and periodontally healthy controls. J Clin Periodontol. 2005. 32:238–243.
Article7. Ellis SD, Tucci MA, Serio FG, Johnson RB. Factors for progression of periodontal diseases. J Oral Pathol Med. 1998. 27:101–105.
Article8. Tulunoglu O, Alacam A, Bastug M, Yavuzer S. Superoxide dismutase activity in healthy and inflamed pulp tissues of permanent teeth in children. J Clin Pediatr Dent. 1998. 22:341–345.9. Chapple IL, Mason GI, Garner I, Matthews JB, Thorpe GH, Maxwell SR, et al. Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum, saliva and crevicular fluid. Ann Clin Biochem. 1997. 34(Pt 4):412–421.
Article10. Chapple IL, Brock G, Eftimiadi C, Matthews JB. Glutathione in gingival crevicular fluid and its relation to local antioxidant capacity in periodontal health and disease. Mol Pathol. 2002. 55:367–373.
Article11. Rao RK, Thomas DW, Pepperl S, Porreca F. Salivary epidermal growth factor plays a role in protection of ileal mucosal integrity. Dig Dis Sci. 1997. 42:2175–2181.12. Liskmann S, Vihalemm T, Salum O, Zilmer K, Fischer K, Zilmer M. Characterization of the antioxidant profile of human saliva in peri-implant health and disease. Clin Oral Implants Res. 2007. 18:27–33.
Article13. Na HJ, Kim OS, Park BJ. Expression of superoxide dismutase isoforms in inflamed gingiva. J Korean Acad Periodontol. 2006. 36:97–112.
Article14. Moore S, Calder KA, Miller NJ, Rice-Evans CA. Antioxidant activity of saliva and periodontal disease. Free Radic Res. 1994. 21:417–425.
Article15. Battino M, Bullon P, Wilson M, Newman H. Oxidative injury and inflammatory periodontal diseases: the challenge of anti-oxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med. 1999. 10:458–476.
Article16. Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci (Lond). 2003. 105:167–172.
Article17. Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004. 31:515–521.
Article18. Cutando A, Galindo P, Gomez-Moreno G, Arana C, Bolanos J, Acuna-Castroviejo D, et al. Relationship between salivary melatonin and severity of periodontal disease. J Periodontol. 2006. 77:1533–1538.
Article19. Pussinen PJ, Laatikainen T, Alfthan G, Asikainen S, Jousilahti P. Periodontitis is associated with a low concentration of vitamin C in plasma. Clin Diagn Lab Immunol. 2003. 10:897–902.
Article20. Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM, et al. Lipid peroxidation: a possible role in the induction and progression of chronic periodontitis. J Periodontal Res. 2005. 40:378–384.
Article21. Canakci V, Yildirim A, Canakci CF, Eltas A, Cicek Y, Canakci H. Total antioxidant capacity and antioxidant enzymes in serum, saliva, and gingival crevicular fluid of preeclamptic women with and without periodontal disease. J Periodontol. 2007. 78:1602–1611.
Article22. Ozmeric N, Elgun S, Uraz A. Salivary arginase in patients with adult periodontitis. Clin Oral Investig. 2000. 4:21–24.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effectiveness of Subgingival Scaling and Root Planing via Closed Approach in Calculus Removal
- The Clinical and Microbiological Study of the Effect of Minocycline Strip Locally Administrated on Adult Periodontitis
- Evaluation of the wear of the periodontal curet's cutting edge
- Gingival color change after scaling & subgingival root planing
- Association of gingival biotype with the results of scaling and root planing