Diagnosis and Treatment of Latent Tuberculosis Infection
- Affiliations
-
- 1Division of Pulmonary, Sleep, and Critical Care Medicine, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. lee-sh@korea.ac.kr
- KMID: 2320595
- DOI: http://doi.org/10.4046/trd.2015.78.2.56
Abstract
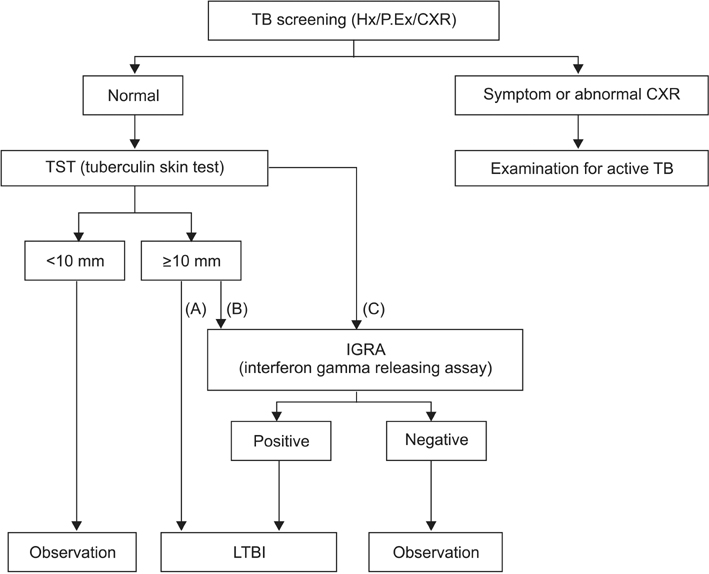
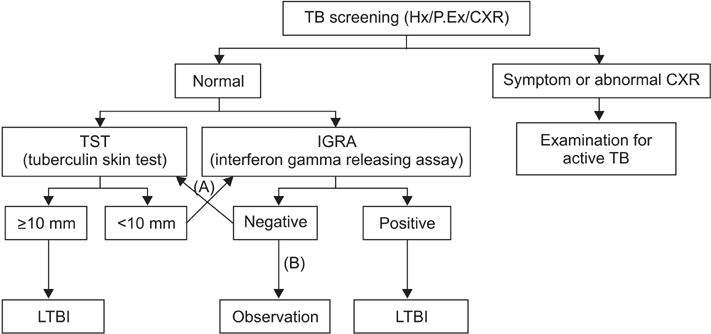
- A small number of viable tuberculosis bacilli can reside in an individual with latent tuberculosis infection (LTBI) without obvious clinical symptoms or abnormal chest radiographs. Diagnosis and treatment for LTBI are important for tuberculosis (TB) control in public and private health, especially in high-risk populations. The updated 2014 Korean guidelines for TB recommend that tuberculin skin tests, interferon-gamma release assays, or a combination of the two can be used for LTBI diagnosis according to age and immune status of the host as well as TB contact history. The regimens for LTBI treatment include isoniazid, rifampicin, or isoniazid/rifampicin. However, results of drug susceptibility test from the index case must be considered in selecting the appropriate drug for recent contacts. Standardized LTBI diagnosis and treatment based on the new 2014 guidelines will contribute to the effective TB control in Korea as well as to the establishment of updated guidelines.
MeSH Terms
Figure
Cited by 5 articles
-
Incidence of Active Tuberculosis within One Year after Tumor Necrosis Factor Inhibitor Treatment according to Latent Tuberculosis Infection Status in Patients with Inflammatory Bowel Disease
Jieun Kang, Dae Hyun Jeong, Minkyu Han, Suk-Kyun Yang, Jeong-Sik Byeon, Byong Duk Ye, Sang Hyoung Park, Sung Wook Hwang, Tae Sun Shim, Kyung-Wook Jo
J Korean Med Sci. 2018;33(47):. doi: 10.3346/jkms.2018.33.e292.Prevalence and Risk Factors of Latent Tuberculosis Infection among Healthcare Workers
Sunhong Lee, Ye Li Lee, Yong Chan Kim, Eun Jin Kim, Jung Yeon Heo, Young Hwa Choi
Korean J Healthc Assoc Infect Control Prev. 2019;24(2):52-59. doi: 10.14192/kjicp.2019.24.2.52.Pulmonary Tuberculosis Diagnosis: Where We Are?
Hamed Ebrahimzadeh Leylabadlo, Hossein Samadi Kafil, Mehdi Yousefi, Mohammad Aghazadeh, Mohammad Asgharzadeh
Tuberc Respir Dis. 2016;79(3):134-142. doi: 10.4046/trd.2016.79.3.134.The Prevalence and Risk Factors of Latent Tuberculosis Infection among Health Care Workers Working in a Tertiary Hospital in South Korea
Jae Seuk Park
Tuberc Respir Dis. 2018;81(4):274-280. doi: 10.4046/trd.2018.0020.Pre-immigration Screening for Tuberculosis in South Korea: A Comparison of Smear- and Culture-Based Protocols
Sangyoon Lee, Ji Young Ryu, Dae-Hwan Kim
Tuberc Respir Dis. 2019;82(2):151-157. doi: 10.4046/trd.2018.0009.
Reference
-
1. Sutherland I. Recent studies in the epidemiology of tuberculosis, based on the risk of being infected with tubercle bacilli. Adv Tuberc Res. 1976; 19:1–63.2. Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003; 362:887–899.3. World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization;2014.4. Joint Committee for the Development of Korean Guidelines for Tuberculosis, Korean Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. Seoul: Korean Centers for Disease Control and Prevention;2011.5. Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korean Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Seoul and Cheongwon: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention;2014.6. Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998; 2:27–36.7. Choi CM, Kang CI, Kim DH, Kim CH, Kim HJ, Lee CH, et al. The role of TST in the diagnosis of latent tuberculosis infection among military personnel in South Korea. Int J Tuberc Lung Dis. 2006; 10:1342–1346.8. Lee JY, Choi HJ, Park IN, Hong SB, Oh YM, Lim CM, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006; 28:24–30.9. Lee SW, Oh SY, Lee JB, Choi CM, Kim HJ. Tuberculin skin test distribution following a change in BCG vaccination policy. PLoS One. 2014; 9:e86419.10. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000; 161(4 Pt 2):S221–S247.11. Grzybowski S, Fishaut H, Rowe J, Brown A. Tuberculosis among patients with various radiologic abnormalities, followed by the chest clinic service. Am Rev Respir Dis. 1971; 104:605–608.12. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000; 161(4 Pt 1):1376–1395.13. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005; 293:2756–2761.14. Song S, Jeon D, Kim JW, Kim YD, Kim SP, Cho JS, et al. Performance of confirmatory interferon-gamma release assays in school TB outbreaks. Chest. 2012; 141:983–988.15. Kim HJ, Lee GH, Ryoo S, Oh SY, Lee JB, Kim JH, et al. Role of confirmatory interferon-gamma release assays in school outbreaks of tuberculosis in South Korea. Int J Tuberc Lung Dis. 2015; Forthcoming.16. Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010; 36:1185–1206.17. National Institute for Health and Care Excellence. Clinical guideline 33. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control [Internet]. London: National Institute for Health and Care Excellence;2011. cited 2014 Dec 12. Available from: http://www.nice.org.uk/CG117.18. Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med. 2013; 187:206–211.19. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999; 159:15–21.20. Kim SY, Park MS, Kim YS, Kim SK, Chang J, Yong D, et al. Tuberculin skin test and boosted reactions among newly employed healthcare workers: an observational study. PLoS One. 2013; 8:e64563.21. World Health Organization. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children. 2nd ed. Geneva: World Health Organization;2014.22. Canadian Thoracic Society, The Pulblic Health Agency of Canada and Licensors. Canadian tuberculosis standards. 7th ed. Ottawa: Pulblic Health Agency of Canada;2014.23. American Academy of Pediatrics. Tuberculosis. In : Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Disease. Elk Grove Village, IL: American Academy of Pediatrics;2012. p. 736–759.24. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection: United States, 2010. MMWR Recomm Rep. 2010; 59:1–25.25. National Tuberculosis Controllers Association. Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005; 54:1–47.26. Lobue P, Menzies D. Treatment of latent tuberculosis infection: an update. Respirology. 2010; 15:603–622.27. Lee SH, Yim JJ, Kim HJ, Shim TS, Seo HS, Cho YS, et al. Adverse events and development of tuberculosis after 4 months of rifampicin prophylaxis in a tuberculosis outbreak. Epidemiol Infect. 2012; 140:1028–1035.28. Yun JW, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Diagnosis and treatment of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. J Korean Med Sci. 2007; 22:779–783.29. Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F, et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis. 2007; 45:715–722.30. Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011; 365:2155–2166.31. Jung YJ, Lee JY, Jo KW, Yoo B, Lee CK, Kim YG, et al. The 'either test positive' strategy for latent tuberculous infection before anti-tumour necrosis factor treatment. Int J Tuberc Lung Dis. 2014; 18:428–434.32. Gomez-Reino JJ, Carmona L, Angel Descalzo M. Biobadaser Group. Risk of tuberculosis in patients treated with tumor necrosis factor antagonists due to incomplete prevention of reactivation of latent infection. Arthritis Rheum. 2007; 57:756–761.33. British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax. 2005; 60:800–805.34. Mariette X, Salmon D. French guidelines for diagnosis and treating latent and active tuberculosis in patients with RA treated with TNF blockers. Ann Rheum Dis. 2003; 62:791.35. Shim TS. Diagnosis and treatment of latent tuberculosis infection due to initiation of anti-TNF therapy. Tuberc Respir Dis. 2014; 76:261–268.36. Kim SH, Lee SO, Park JB, Park IA, Park SJ, Yun SC, et al. A prospective longitudinal study evaluating the usefulness of a T-cell-based assay for latent tuberculosis infection in kidney transplant recipients. Am J Transplant. 2011; 11:1927–1935.37. Kim SY, Jung GS, Kim SK, Chang J, Kim MS, Kim YS, et al. Comparison of the tuberculin skin test and interferon-gamma release assay for the diagnosis of latent tuberculosis infection before kidney transplantation. Infection. 2013; 41:103–110.38. Kim SH, Lee SO, Park IA, Park SJ, Choi SH, Kim YS, et al. Diagnostic usefulness of a T cell-based assay for latent tuberculosis infection in kidney transplant candidates before transplantation. Transpl Infect Dis. 2010; 12:113–119.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and treatment of latent tuberculosis infection
- Treatment of latent tuberculous infection in children and adolescent
- Diagnosis and Treatment of Latent Tuberculosis Infection for Healthcare Workers
- Diagnosis and treatment of latent tuberculosis infection
- Tuberculosis Infection and Latent Tuberculosis