Tuberc Respir Dis.
2014 Apr;76(4):163-168.
Usefulness of Sputum Induction with Hypertonic Saline in a Real Clinical Practice for Bacteriological Yields of Active Pulmonary Tuberculosis
- Affiliations
-
- 1Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea. doc4u@hanmail.net
- 2Department of Otorhinolaryngology, Jeju National University School of Medicine, Jeju, Korea.
Abstract
- BACKGROUND
Mycobacterial identification in active pulmonary tuberculosis (APTB) is confirmative, even though successful rates using self-expectorated sputum are limited. Sputum specimens collected by hypertonic saline nebulization showed higher bacteriologic diagnostic sensitivities over those of self-expectoration, mostly studied in smear-negative or sputum-scarce patients. The efficacy of induced sputum was rarely assessed in real clinical settings.
METHODS
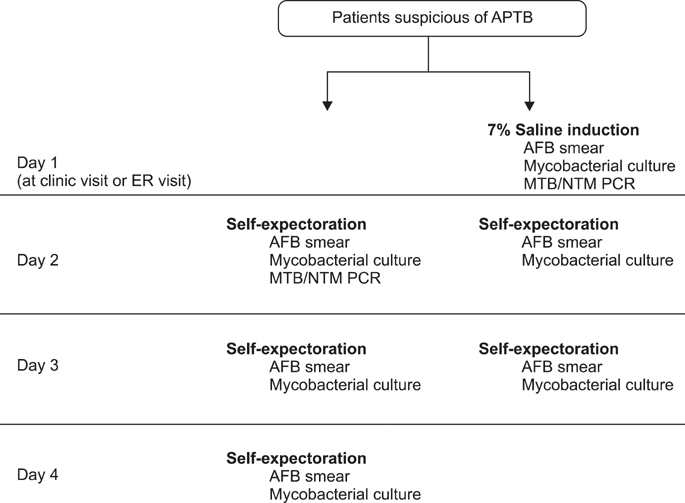
A prospective randomized case-control study was performed in one hospital. The subjects highly suspicious of APTB were asked to provide 3 pairs of sputum specimens in 3 consecutive days. The first pairs of the specimens were obtained either by self-expectoration (ES) from the next day of the visit or sputum induction with 7% saline nebulization in clinic (SI), and the other specimens were collected in the same way. The samples were tested in microscopy, culture, and polymerase chain reaction (PCR). The outcomes of the bacteriological diagnosis were compared.
RESULTS
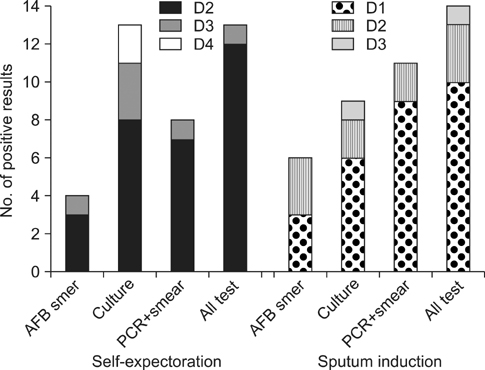
Seventy six patients were assigned to either ES (38 subjects, median age of 51, 65.8% male) or SI (38 subjects, median age of 55, 52.6% male). APTB was clinically confirmed in 51 patients (70.8%), 27 in ES and 24 in SI. Among the APTB, more adequate specimens were collected from SI (41/65, 63.1%) than ES (34/80, 42.5%) (p=0.01). Bacteriological confirmation was achieved in 14 (58.3%) patients in SI, and 13 (48.1%) in ES (p=0.46). In the same-day bacteriological diagnosis with microscopy and PCR, there were positive results for 9 patients (37.5%) in SI and 7 patients (25.9%) in ES (p=0.37).
CONCLUSION
Sputum induction improves sputum specimen adequacy. It may be useful for the same-day bacteriological diagnosis with microscopic examination and PCR.
MeSH Terms
Figure
Reference
-
1. World Health Organization. Global tuberculosis report 2012. Geneva: World Health Organization;2012.2. Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013; 13:349–361.3. Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med. 2013; 368:745–755.4. Raviglione MC, O'Brien RJ. Tuberculosis. In : Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's principles of internal medicine. 18th ed. New York: McGraw-Hill;2012. p. 1340–1359.5. Pitchenik AE, Ganjei P, Torres A, Evans DA, Rubin E, Baier H. Sputum examination for the diagnosis of Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986; 133:226–229.6. Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE, et al. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. 1992; 47:25–29.7. Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006; 354:241–250.8. Al Zahrani K, Al Jahdali H, Poirier L, Rene P, Menzies D. Yield of smear, culture and amplification tests from repeated sputum induction for the diagnosis of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2001; 5:855–860.9. Na MJ. Comparison of induced sputum and bronchoscopy in diagnosis of active pulmonary tuberculosis. Korean J Med. 1998; 55:75–82.10. Saglam L, Akgun M, Aktas E. Usefulness of induced sputum and fibreoptic bronchoscopy specimens in the diagnosis of pulmonary tuberculosis. J Int Med Res. 2005; 33:260–265.11. Biswas S, Das A, Sinha A, Das SK, Bairagya TD. The role of induced sputum in the diagnosis of pulmonary tuberculosis. Lung India. 2013; 30:199–202.12. Hepple P, Ford N, McNerney R. Microscopy compared to culture for the diagnosis of tuberculosis in induced sputum samples: a systematic review. Int J Tuberc Lung Dis. 2012; 16:579–588.13. Anderson C, Inhaber N, Menzies D. Comparison of sputum induction with fiber-optic bronchoscopy in the diagnosis of tuberculosis. Am J Respir Crit Care Med. 1995; 152(5 Pt 1):1570–1574.14. Brown M, Varia H, Bassett P, Davidson RN, Wall R, Pasvol G. Prospective study of sputum induction, gastric washing, and bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in patients who are unable to expectorate. Clin Infect Dis. 2007; 44:1415–1420.15. Murray PR, Washington JA. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc. 1975; 50:339–344.16. Lee J. Formation of airway mucus: synthesis, exocytosis and dilution of gel-forming mucins. Korean J Asthma Allergy Clin Immunol. 2012; 32:73–80.17. Abdullah LH, Bundy JT, Ehre C, Davis CW. Mucin secretion and PKC isoforms in SPOC1 goblet cells: differential activation by purinergic agonist and PMA. Am J Physiol Lung Cell Mol Physiol. 2003; 285:L149–L160.18. Irwin RS, Curley FJ, Bennett FM. Appropriate use of antitussives and protussives: a practical review. Drugs. 1993; 46:80–91.19. Atiq-ur-Rehman M, Naseem A, Hussain T. Comparison of diagnostic yield of AFB with sputum induction to spontaneous sputum examination in suspected pulmonary tuberculosis. J Coll Physicians Surg Pak. 2009; 19:506–509.20. Bell D, Leckie V, McKendrick M. The role of induced sputum in the diagnosis of pulmonary tuberculosis. J Infect. 2003; 47:317–321.21. Peter JG, Theron G, Pooran A, Thomas J, Pascoe M, Dheda K. Comparison of two methods for acquisition of sputum samples for diagnosis of suspected tuberculosis in smear-negative or sputum-scarce people: a randomised controlled trial. Lancet Respir Med. 2013; 1:471–478.22. Oh MS, Lee J. The increase of nontuberculous mycobacterial isolation in the specimens from respiratory system in Jeju. Ann Clin Microbiol. 2013; 16:13–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Induced Sputum and Bronchoscopy in Diagnosis of Active Pulmonary Tuberculosis
- The Usefulness of 99mTc-MIBI in the Detection of Active Pulmonary Tuberculosis
- Pulmonary Langerhans Cell Histiocytosis Accompanied by Active Pulmonary Tuberculosis
- The Diagnostic Significance of 67 Gallium Lung Scan and High Resolution Computed Tomography in Patients with Pulmonary Tuberculosis
- Two Cases of Pharyngeal Tuberculosis Secondary to Pulmonary Tuberculosis