Tuberc Respir Dis.
2012 Jan;72(1):44-49.
Evaluation of Reverse Hybridization Assay for Detecting Fluoroquinolone and Kanamycin Resistance in Multidrug-Resistance Mycobacterium tuberculosis Clinical Isolates
- Affiliations
-
- 1Masan National Hospital, Changwon, Korea. 0120pcs@hanmail.net
Abstract
- BACKGROUND
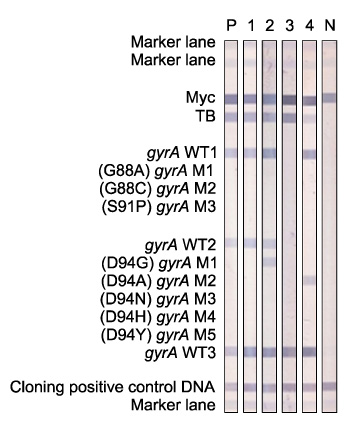
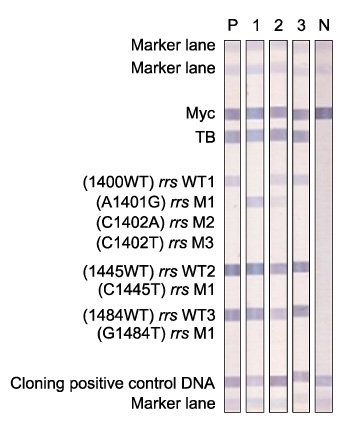
Multidrug-resistant tuberculosis (MDR-TB) is an increasing public health problem and poses a serious threat to global TB control. Fluoroquinolone (FQ) and aminoglycoside (AG) are essential anti-TB drugs for MDR-TB treatment. REBA MTB-FQ(R) and REBA MTB-KM(R) (M&D, Wonju, Korea) were evaluated for rapid detection of FQ and kanamycin (KM) resistance in MDR-TB clinical isolates.
METHODS
M. tuberculosis (n=67) were isolated and cultured from the sputum samples of MDR-TB patients for extracting DNA of the bacilli. Mutations in genes, gyrA and rrs, that have been known to be associated with resistance to FQ and KM were analyzed using both REBA MTB-FQ(R) and REBA MTB-KM(R), respectively. The isolates were also utilized for a conventional phenotypic drug susceptibility test (DST) as the gold standard of FQ and KM resistance. The molecular and phenotypic DST results were compared.
RESULTS
Sensitivity and specificity of REBA MTB-FQ(R) were 77 and 100%, respectively. Positive predictive value and negative predictive value of the assay were 100 and 95%, respectively, for FQ resistance. Sensitivity, specificity, positive predictive value and negative predictive value of REBA MTB-KM(R) for detecting KM resistance were 66%, 94%, 70%, and 95%, respectively.
CONCLUSION
REBA MTB-FQ(R) and REBA MTB-KM(R) evaluated in this study showed excellent specificities as 100 and 94%, respectively. However, sensitivities of the assays were low. It is essential to increase sensitivity of the rapid drug resistance assays for appropriate MDR-TB treatment, suggesting further investigation to detect new or other mutation sites of the associated genes in M. tuberculosis is required.
Keyword
MeSH Terms
Figure
Reference
-
1. Multidrug and extensively drug-resistant Tb (m/xdr-Tb): 2010 global report on surveillance and response. 2010. Geneva, Switzerland: World Health Organization.2. Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007. 11:571–576.3. Jeon D, Shin D, Kang H, Sung N, Kweon K, Shin E, et al. Trend of multidrug and extensively drug resistant tuberculosis in a tuberculosis referral hospital, 2001~2005. Tuberc Respir Dis. 2008. 64:187–193.4. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003. 167:603–662.5. Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008. 2008. Geneva, Switzerland: World Health Organization.6. Joh JS, Lee CH, Lee JE, Park YK, Bai GH, Kim EC, et al. The interval between initiation of anti-tuberculosis treatment in patients with culture-positive pulmonary tuberculosis and receipt of drug-susceptibility test results. J Korean Med Sci. 2007. 22:26–29.7. Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J. 2005. 25:564–569.8. Takiff HE, Salazar L, Guerrero C, Philipp W, Huang WM, Kreiswirth B, et al. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob Agents Chemother. 1994. 38:773–780.9. Cheng AF, Yew WW, Chan EW, Chin ML, Hui MM, Chan RC. Multiplex PCR amplimer conformation analysis for rapid detection of gyrA mutations in fluoroquinolone-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother. 2004. 48:596–601.10. Giannoni F, Iona E, Sementilli F, Brunori L, Pardini M, Migliori GB, et al. Evaluation of a new line probe assay for rapid identification of gyrA mutations in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005. 49:2928–2933.11. Suzuki Y, Katsukawa C, Tamaru A, Abe C, Makino M, Mizuguchi Y, et al. Detection of kanamycin-resistant Mycobacterium tuberculosis by identifying mutations in the 16S rRNA gene. J Clin Microbiol. 1998. 36:1220–1225.12. Krüüner A, Jureen P, Levina K, Ghebremichael S, Hoffner S. Discordant resistance to kanamycin and amikacin in drug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003. 47:2971–2973.13. Maus CE, Plikaytis BB, Shinnick TM. Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005. 49:3192–3197.14. Jugheli L, Bzekalava N, de Rijk P, Fissette K, Portaels F, Rigouts L. High level of cross-resistance between kanamycin, amikacin, and capreomycin among Mycobacterium tuberculosis isolates from Georgia and a close relation with mutations in the rrs gene. Antimicrob Agents Chemother. 2009. 53:5064–5068.15. Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969. 41:21–43.16. Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston MJ, et al. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993. 341:647–650.17. Musser JM. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin Microbiol Rev. 1995. 8:496–514.18. Wade MM, Zhang Y. Mechanisms of drug resistance in Mycobacterium tuberculosis. Front Biosci. 2004. 9:975–994.19. Tsukamura M, Nakamura E, Yoshii S, Amano H. Therapeutic effect of a new antibacterial substance ofloxacin (DL8280) on pulmonary tuberculosis. Am Rev Respir Dis. 1985. 131:352–356.20. Sullivan EA, Kreiswirth BN, Palumbo L, Kapur V, Musser JM, Ebrahimzadeh A, et al. Emergence of fluoroquinolone-resistant tuberculosis in New York City. Lancet. 1995. 345:1148–1150.21. Montero C, Mateu G, Rodriguez R, Takiff H. Intrinsic resistance of Mycobacterium smegmatis to fluoroquinolones may be influenced by new pentapeptide protein MfpA. Antimicrob Agents Chemother. 2001. 45:3387–3392.22. Drlica K, Malik M. Fluoroquinolones: action and resistance. Curr Top Med Chem. 2003. 3:249–282.23. Hillemann D, Rüsch-Gerdes S, Richter E. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin-capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol. 2009. 47:1767–1772.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early Detection of Mycobacterium Tuberculosis and Determination Rifampin-Resistance by PCR-Reverse Hybridization (Line Probe Assay) v
- Trend of multidrug and fluoroquinolone resistance in Mycobacterium tuberculosis isolates from 2010 to 2014 in Korea: a multicenter study
- Molecular Detection of Fluoroquinolone Resistance in Multidrug-Resistant Mycobacterium tuberculosis Isolates
- The Drug Resistance of Mycobacterium tuberculosis in Seongnam
- Detection of Clarithromycin-resistant Strains from Clinical Isolates of Mycobacterium abscessus