Tuberc Respir Dis.
2009 Feb;66(2):104-109.
Efficacy of Darbepoetin alfa in Anemia Developed during Chemotherapy for Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. cyberkks@chonnam.ac.kr
Abstract
- BACKGROUND
Anemia is quite common in lung cancer patients and known to decrease the quality of life. Darbepoetin alfa is an erythropoiesis-stimulating protein approved for administration to cancer patients. This study examined the efficacy and safety of darbepoetin alfa in lung cancer patients with a hemoglobin concentration <10 g/dl during chemotherapy.
METHODS
Lung cancer patients (n=178) received darbepoetin alfa at doses of 1.91 microgram/kg per week until the hemoglobin concentration increased to >10 g/dl. The efficacy and safety were measured by comparing the hemoglobin concentration and assessing the adverse events.
RESULTS
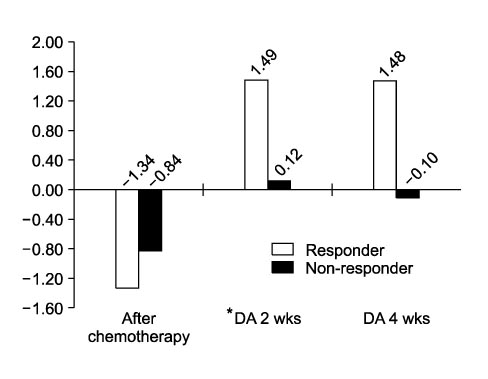
After chemotherapy, the hemoglobin concentration decreased to 9.03+/-0.64 g/dl. With the darbepoetin alfa treatment, the hemoglobin concentration increased to 10.09+/-1.17 g/dl after 4 weeks reaching a peak hemoglobin concentration of 10.45+/-1.18 g/dl. The changes in hemoglobin after 4 and 8 weeks with treatment were 1.08+/-1.24 g/dl and 1.38+/-1.59 g/dl (p<0.01). At least a 1 g/dl or more increase in hemoglobin was observed in 62.4% of patients. There were no serious adverse effects except for some mild reactions.
CONCLUSION
Darbepoetin alfa administered to lung cancer patients appears to be an effective, well-tolerated treatment for chemotherapy induced anemia.
Keyword
MeSH Terms
Figure
Reference
-
1. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999. 91:1616–1634.2. Scagliotti GV, Novello S. Role of erythropoietin in the treatment of lung cancer associated anaemia. Lung Cancer. 2001. 34:Suppl 4. S91–S94.3. Lyman GH, Berndt ER, Kallich JD, Erder MH, Crown WH, Long SR, et al. The economic burden of anemia in cancer patients receiving chemotherapy. Value Health. 2005. 8:149–156.4. Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. Second of two parts--blood conservation. N Engl J Med. 1999. 340:525–533.5. Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001. 19:2875–2882.6. Demetri GD, Kris M, Wade J, Degos L, Cella D. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J Clin Oncol. 1998. 16:3412–3425.7. Cella D, Zagari MJ, Vandoros C, Gagnon DD, Hurtz HJ, Nortier JW. Epoetin alfa treatment results in clinically significant improvements in quality of life in anemic cancer patients when referenced to the general population. J Clin Oncol. 2003. 21:366–373.8. Cella D, Kallich J, McDermott A, Xu X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Ann Oncol. 2004. 15:979–986.9. Berndt E, Kallich J, McDermott A, Xu X, Lee H, Glaspy J. Reductions in anaemia and fatigue are associated with improvements in productivity in cancer patients receiving chemotherapy. Pharmacoeconomics. 2005. 23:505–514.10. Patton J, Reeves T, Wallace J. Effectiveness of darbepoetin alfa versus epoetin alfa in patients with chemotherapy-induced anemia treated in clinical practice. Oncologist. 2004. 9:451–458.11. Waltzman R, Croot C, Justice GR, Fesen MR, Charu V, Williams D. Randomized comparison of epoetin alfa (40,000 U weekly) and darbepoetin alfa (200 microg every 2 weeks) in anemic patients with cancer receiving chemotherapy. Oncologist. 2005. 10:642–650.12. Erythropoiesis Stimulating Agents (ESA) [Aranesp (darbepoetin), Epogen (epoetin alfa), and Procrit (epoe tin alfa)]. Information for Healthcare Professionals [Internet]. 2007. 02. 16. cited 2007 Mar 21. Silver Spring: U.S. Food and Drug Administration/Center for Drug Evaluation and Research;Available from: http://www.fda.gov/cder/drug/InfoSheets/HCP/RHE2007HCP.htm.13. Senecal FM, Yee L, Gabrail N, Charu V, Tomita D, Rossi G, et al. Treatment of chemotherapy-induced anemia in breast cancer: results of a randomized controlled trial of darbepoetin alfa 200 microg every 2 weeks versus epoetin alfa 40,000 U weekly. Clin Breast Cancer. 2005. 6:446–454.14. Aranesp (darbepoetin alfa) [package insert]. 2008. 03. Thousand oaks, CA: Amgen Inc..15. Procrit (epoetin alfa) [package insert]. 2008. 03. Raritan, NJ: OrthoBiotech. L.P..16. Lastiri JM, Specterman SR, Rendo P, Pallotta MG, Varela MS, Goldstein S. Predictive response variables to recombinant human erythropoietin treatment in patients with anemia and cancer. Medicina (B Aires). 2002. 62:41–47.17. Rodgers GM. Clinical practice guidelines in oncology: cancer and treatment-related anemia [Internet]. 2005. cited 2005 Dec 10. Fort Washington: National Comprehensive Cancer Network;Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Randomized, Controlled Trial of Darbepoetin Alfa for the Treatment of Renal Anemia in Hemodialysis Patients
- Hemoglobin Variability Associated with Different Erythropoiesis Stimulating Agents in Hemodialysis Patients
- Effectiveness of darbepoetin alfa in multiple myeloma patients receiving chemotherapy including novel agents
- Efficacy and cost-effectiveness of darbepoetin alfa once every 4 weeks versus continuous erythropoietin receptor activator once every 4 weeks for anemia correction in patients with chronic kidney disease not on dialysis
- Comparison of Erythropoietic Effect between Epoetin-alpha and Darbepoetin-alpha in Hemodialysis Patients: A Randomized Crossover Study