Tuberc Respir Dis.
2008 Dec;65(6):464-470.
Presumptive Role of Neutrophilic Oxidative Stress in Oxygen-induced Acute Lung Injury in Rats
- Affiliations
-
- 1Department of Anatomy, Catholic University of Daegu, School of Medicine, Daegu, Korea.
- 2Department of Physiology, Catholic University of Daegu, School of Medicine, Daegu, Korea. leeym@cu.ac.kr
Abstract
-
BACKGROUND: This study examined the role of neutrophilc oxidative stress in an O2-induced acute lung injury (ALI).
METHODS
For 48 h, experimental rats were exposed to pure oxygen (normobaric hyperoxia) in a plastic cage. Forty-eight hours after O2 breathing, the rats were sacrificed and the parameters for ALI associated with neutrophilic oxidative stress were assessed.
RESULTS
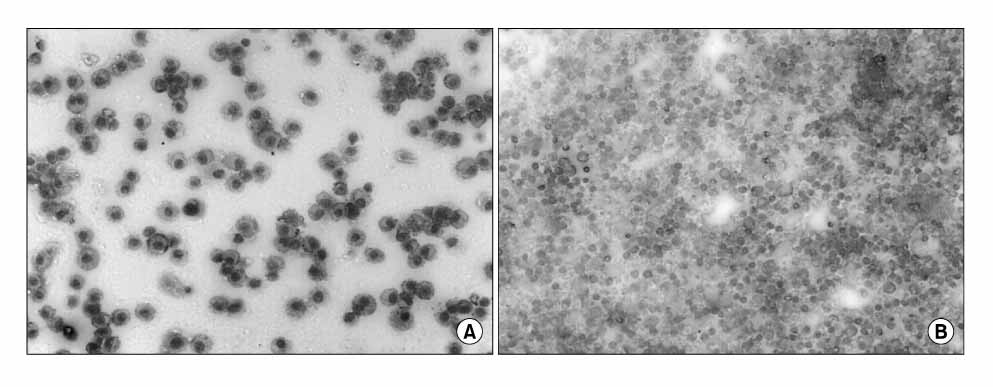
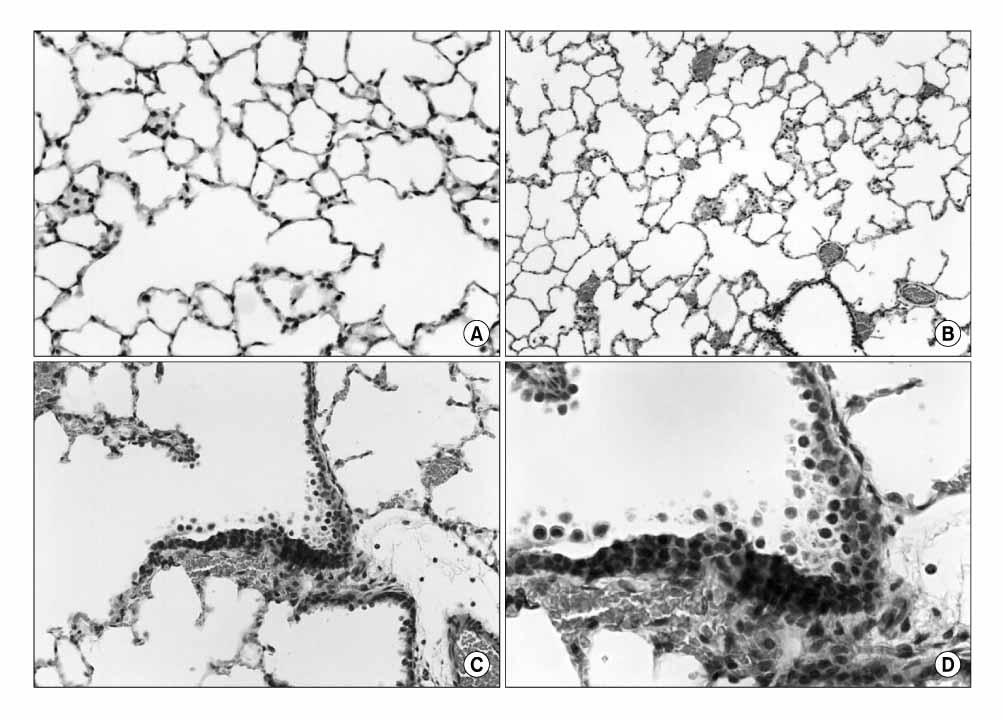
Normobaric pure oxygen induced ALI, which was quite similar to ARDS. The O2-induced neutrophilic oxidative stress was identified by confirming of the increase in lung myeloperoxidase, BAL neutrophils, malondialdehyde (MDA), cytosolic phospholipase A2 (cPLA2) activity in the lung, histological changes and BAL cytospin morphology.
CONCLUSION
In part, ALI-caused by oxygen is affected by neutrophils especially by the generation of free radicals.
Keyword
MeSH Terms
Figure
Reference
-
1. Hay WW Jr, Bell EF. Oxygen therapy, oxygen toxicity, and STOP-ROP trial. Pediatrics. 2000. 105:424–425.2. Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980. 122:123–143.3. Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998. 11:745–757.4. Guidot DM, Repine JE, Kitlowski AD, Flores SC, Nelson SK, Wright RM, et al. Mitochondrial respiration scavenges extramitochondrial superoxide anion via a nonenzymatic mechanism. J Clin Invest. 1995. 96:1131–1136.5. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000. 342:1334–1349.6. Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989. 180:136–139.7. Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985. 59:1978–1985.8. Carraway MS, Piantadosi CA, Jenkinson CP, Huang YC. Differential expression of arginase and iNOS in the lung in sepsis. Exp Lung Res. 1998. 24:253–268.9. Katsumata M, Gupta C, Goldman AS. A rapid assay for activity of phospholipase A2 using radioactive substrate. Anal Biochem. 1986. 154:676–681.10. Capellier G, Maupoil V, Boussat S, Laurent E, Neidhardt A. Oxygen toxicity and tolerance. Minerva Anestesiol. 1999. 65:388–392.11. Torbati D, Tan GH, Smith S, Frazier KS, Gelvez J, Fakioglu H, et al. Multiple-organ effect of normobaric hyperoxia in neonatal rats. J Crit Care. 2006. 21:85–93.12. Roman K, Vladimíra M, Alexander C. Highly specific, simple and rapid method for the determination of malondialdehyde in blood using high-performance liquid chromatography. Clin Chem Lab Med. 2002. 40:1032–1035.13. Sukhotnik I, Coran AG, Greenblatt R, Brod V, Mogilner J, Shiloni E, et al. Effect of 100% oxygen on E-selectin expression, recruitment of neutrophils and enterocyte apoptosis following intestinal ischemia-reperfusion in a rat. Pediatr Surg Int. 2008. 24:29–35.14. Shvedova AA, Kisin ER, Murray AR, Kommineni C, Castranova V, Fadeel B, et al. Increased accumulation of neutrophils and decreased fibrosis in the lung of NADPH oxidase-deficient C57BL/6 mice exposed to carbon nanotubes. Toxicol Appl Pharmacol. 2008. 231:235–240.15. Shasby DM, Fox RB, Harada RN, Repine JE. Reduction of the edema of acute hyperoxic lung injury by granulocyte depletion. J Appl Physiol. 1982. 52:1237–1244.16. Hybertson BM, Lee YM, Repine JE. Phagocytes and acute lung injury: dual roles for interleukin-1. Ann N Y Acad Sci. 1997. 832:266–273.17. Lin Y, Jamieson D. Role of neutrophils in hyperbaric and normobaric oxygen toxicity. Pathophysiology. 1995. 2:9–16.18. Prudhomme JB, Ware LB. Acute lung injury and acute respiratory distress syndrome: mechanisms and potential new therapies. Drug Discov Today Dis Mech. 2004. 1:123–128.19. Lee YM, Hybertson BM, Terada LS, Repine AJ, Cho HG, Repine JE. Mepacrine decreases lung leak in rats given interleukin-1 intratracheally. Am J Respir Crit Care Med. 1997. 155:1624–1628.20. Guidot DM. Endotoxin treatment increases lung mitochondrial scavenging of extramitochondrial superoxide in hyperoxia-exposed rats. Arch Biochem Biophys. 1996. 326:266–270.21. Boyer CS, Bannenberg GL, Neve EP, Ryrfeldt A, Moldéus P. Evidence for the activation of the signal-responsive phospholipase A2 by exogenous hydrogen peroxide. Biochem Pharmacol. 1995. 50:753–761.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of the PLA2-Activated Neutrophilic Oxidative Stress in Oleic Acid-Induced Acute Lung Injury
- Phospholipase A2 Contributes to Hemorrhage-induced Acute Lung Injury Through Neutrophilic Respiratory Burst
- Effect of the inhibition of PLA2 on the oxidative stress in the lungs of glutathione depleted rats given endotoxin intratracheally
- Cytosolic Phospholipase A2 Activity in Neutrophilic Oxidative Stress of Platelet-activating Factor-induced Acute Lung Injury
- Effect of the inhibition of platelet activating factor on oxidative lung injury induced by interleukin-1 alpha