Tuberc Respir Dis.
2008 Nov;65(5):379-384.
Comparison of Smear and Culture Positivity using NaOH Method and NALC-NaOH Method for Sputum Treatment
- Affiliations
-
- 1National Masan Tuberculosis Hospital, Masan, Korea. johnofkathy@yahoo.co.kr
- 2International Tuberculosis Research Center, Masan, Korea.
Abstract
-
BACKGROUND: Sputum decontamination with NALC-NaOH (N-acetyl-L-cysteine-sodium hydroxide) is known to better detect Mycobacterium tuberculosis (M. tb) by culture than that with using NaOH, which is widely used in Korean hospitals. In this report, sputum samples collected from pulmonary tuberculosis (TB) patients were treated with either NaOH or NALC-NaOH, and we compared the results of smear and culture positivity to determine whether the NALC-NaOH treatment method improves culture positivity in the sputum samples, and especially for those sputum samples that are smear negative and scanty.
METHODS
For each decontamination method, 436 sputum samples from pulmonary TB patients in the National Masan Tuberculosis Hospital were collected for this study. Sputum from a patient was collected two times for the first and second day of sampling time, and these samples were employed for the decontamination process by performing the 4% NaOH and NALC-2% NaOH treatment methods, respectively, for detecting M. tb by an AFB (Acid Fast Bacilli) smear and also by culture in solid Ogawa medium.
RESULTS
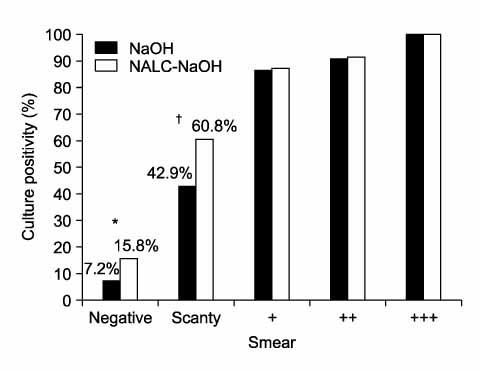
The NaOH and NALC-NaOH treatment methods did not significantly affect the AFB smear positivity of the sputum samples (33.0% vs 39.0%, respectively, p=0.078). However, the culture positive percents of M. tb in the Ogawa medium treated with NALC-NaOH and NaOH were 39.7% and 28.0%, respectively, which was a significantly different (p=0.0003). This difference in culture was more prominent in the sputum samples that were smear negative (the positive percents with NALC-NaOH and NaOH were 15.8% and 7.2%, respectively, p=0.0017) and scanty (NALC-NaOH and NaOH were 60.8% and 42.9%, respectively, p=0.036), but not for a smear that was 1+ or higher (p>0.05).
CONCLUSION
NALC-NaOH treatment is better than NaOH treatment for the detection of M. tb by culture, but not by smear, and especially when the AFB smear is negative and scanty.
Keyword
MeSH Terms
Figure
Reference
-
1. Kent PT, Kubica GP. Public health mycobacteriology: guide for the Level III Laboratory. 1985. 2nd ed. Atlanta: US Department of Health and Human Services, Centers for Disease Control.2. Chang CL, Park TS, Kim MN, Lee NY, Lee HJ, Suh JT. Survey on changes in mycobacterial testing practices in Korean laboratories. Korean J Clin Microbiol. 2001. 4:108–114.3. World Health Organization. Laboratory services in tuberculosis control. Part II. Microscopy. 1998. Geneva: World Health Organization.4. World Health Organization. Laboratory services in tuberculosis control. Part III. Culture. 1998. Geneva: World Health Organization.5. Scott CP, Dos Anjos Filho L, De Queiroz Mello FC, Thornton CG, Bishai WR, Fonseca LS, et al. Comparison of C(18)-carboxypropylbetaine and standard N-acetyl-L-cysteine-NaOH processing of respiratory specimens for increasing tuberculosis smear sensitivity in Brazil. J Clin Microbiol. 2002. 40:3219–3222.6. Yegian D, Budd V. Toxic effect of sodium hydroxide on tubercle bacilli. Am J Clin Pathol. 1952. 22:456–460.7. Ratnam S, March SB. Effect of relative centrifugal force and centrifugation time on sedimentation of mycobacteria in clinical specimens. J Clin Microbiol. 1986. 23:582–585.8. Smithwick RW, Stratigos CB, David HL. Use of cetylpyridinium chloride and sodium chloride for the decontamination of sputum specimens that are transported to the laboratory for the isolation of Mycobacterium tuberculosis. J Clin Microbiol. 1975. 1:411–413.9. Tacquet A, Tison F, Devulder B, Roos P. Techniques for decontamination of pathological specimens for culturing mycobacteria. Bull Int Union Tuberc. 1967. 39:21–24.10. Beam RE, Kim KS, Kubica GP. Comparison of different digestion-decontamination methods for recovery of mycobacteria from sputum: a suggested method for "field processing" of sputa. Scand J Respir Dis. 1967. 48:136–141.11. Sula L. Comparative trials with different decontaminating agents for growing Mycobacterium tuberculosis from sputum specimens. Bull World Health Organ. 1968. 39:647–655.12. Jeong J, Chang CL. Evaluation of mycobacterial recovery by specimen preparation and inoculating media. Korean J Clin Pathol. 2000. 20:188–193.13. Ganoza CA, Ricaldi JN, Chauca J, Rojas G, Munayco C, Agapito J, et al. Novel hypertonic saline-sodium hydroxide (HS-SH) method for decontamination and concentration of sputum samples for Mycobacterium tuberculosis microscopy and culture. J Med Microbiol. 2008. 57:1094–1098.14. Chakravorty S, Dudeja M, Hanif M, Tyagi JS. Utility of universal sample processing methodology, combining smear microscopy, culture, and PCR, for diagnosis of pulmonary tuberculosis. J Clin Microbiol. 2005. 43:2703–2708.15. Thornton CG, MacLellan KM, Brink TL Jr, Lockwood DE, Romagnoli M, Turner J, et al. Novel method for processing respiratory specimens for detection of mycobacteria by using C18-carboxypropylbetaine: blinded study. J Clin Microbiol. 1998. 36:1996–2003.16. Thornton CG, MacLellan KM, Brink TL Jr, Wolfe DM, Llorin OJ, Passen S. Processing respiratory specimens with C18-carboxypropylbetaine: development of a sediment resuspension buffer that contains lytic enzymes to reduce the contamination rate and lecithin to alleviate toxicity. J Clin Microbiol. 1998. 36:2004–2013.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Two Sputum Processing Methods for Detecting Mycobacterium tuberculosis by Culture and PCR: Universal Sample Processing (USP) and NALC-NaOH Methods
- Comparative Evaluation of the Loop-Mediated Isothermal Amplification Assay for Detecting Pulmonary Tuberculosis
- Evaluating the Use of Distilled Water for Washing Sodium Hydroxide in Mycobacterial Culture
- Comparison of Stain Methods with PCR and Culture for the Detection of Mycobacterium Tuberculosis in the Sputum
- Effects of SLA surface treated with NaOH on surface characteristics and response of osteoblast-like cell