Tuberc Respir Dis.
2008 Jun;64(6):466-470.
A Case of Pulmonary Embolism in a Patient with a Factor VII Gene Promoter -401G/A Polymorphism
- Affiliations
-
- 1Department of Internal Medicine, Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea. wichoi@dsmc.or.kr
- 2Department of Immunology, Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
Abstract
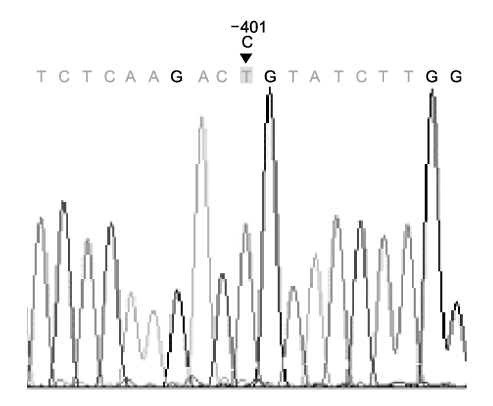
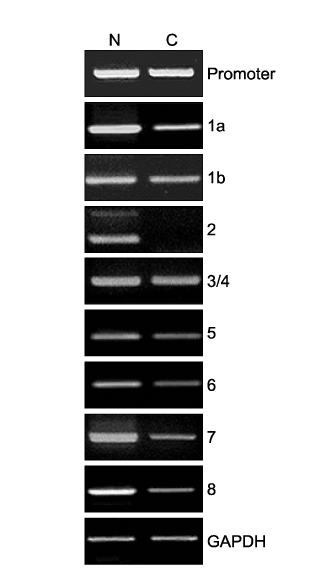
- A factor VII gene -401 G/A polymorphism was identified in a patient with a pulmonary embolism. The patient was a 71-year-old woman who presented with acute-onset dyspnea. A chest CT scan revealed a pulmonary embolism. Despite the administration of low-dose warfarin as anticoagulation therapy, there was an excessively prolonged prothrombin time (PT). The blood tests revealed lower factor VII activity than normal. Full factor VII gene sequencing revealed a G to A substitution at ?401 in the promoter region. There were no other gene sequence anomalies. PCR-based analysis indicated lower factor VII gene expression in the patient than in a control subject. The data suggested the promoter polymorphism to be responsible for the lower transcription level. In conclusion, we encountered a case of Factor VII DNA polymorphism in a patient with a pulmonary embolism showing significantly reduced Factor VII activity.
Keyword
MeSH Terms
Figure
Reference
-
1. Bauer KA. Activation of the factor VII-tissue factor pathway. Thromb Haemost. 1997. 78:108–111.2. O'Hara PJ, Grant FJ, Haldeman BA, Gray CL, Insley MY, Hagen FS, et al. Nucleotide sequence of the gene coding for human factor VII, a vitamin K-dependent protein participating in blood coagulation. Proc Natl Acad Sci U S A. 1987. 84:5158–5162.3. Hong Y, Pedersen NL, Egberg N, de Faire U. Genetic effects for plasma factor VII levels independent of and in common with triglycerides. Thromb Haemost. 1999. 81:382–386.4. Bernardi F, Marchetti G, Pinotti M, Arcieri P, Baroncini C, Papacchini M, et al. Factor VII gene polymorphisms contribute about one third of the factor VII level variation in plasma. Arterioscler Thromb Vasc Biol. 1996. 16:72–76.5. Meade TW, Mellows S, Brozovic M, Miller GJ, Chakrabarti RR, North WR, et al. Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet. 1986. 2:533–537.6. Heywood DM, Carter AM, Catto AJ, Bamford JM, Grant PJ. Polymorphisms of the factor VII gene and circulating FVII:C levels in relation to acute cerebrovascular disease and poststroke mortality. Stroke. 1997. 28:816–821.7. Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). Am J Med. 2002. 113:636–642.8. Freedman MD. Oral anticoagulants: pharmacodynamics, clinical indications and adverse effects. J Clin Pharmacol. 1992. 32:196–209.9. Palareti G, Legnani C. Warfarin withdrawal. Pharmacokinetic-pharmacodynamic considerations. Clin Pharmacokinet. 1996. 30:300–313.10. Carew JA, Pollak ES, High KA, Bauer KA. Severe factor VII deficiency due to a mutation disrupting an Sp1 binding site in the factor VII promoter. Blood. 1998. 92:1639–1645.11. Marchetti G, Patracchini P, Papacchini M, Ferrati M, Bernardi F. A polymorphism in the 5' region of coagulation factor VII gene (F7) caused by an inserted decanucleotide. Hum Genet. 1993. 90:575–576.12. van 't Hooft FM, Silveira A, Tornvall P, Iliadou A, Ehrenborg E, Eriksson P, et al. Two common functional polymorphisms in the promoter region of the coagulation factor VII gene determining plasma factor VII activity and mass concentration. Blood. 1999. 93:3432–3441.13. McVey JH, Boswell E, Mumford AD, Kemball-Cook G, Tuddenham EG. Factor VII deficiency and the FVII mutation database. Hum Mutat. 2001. 17:3–17.14. Taube J, Halsall D, Baglin T. Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over-anticoagulation in patients on long-term treatment. Blood. 2000. 96:1816–1819.15. Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005. 352:2285–2293.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasminogen activator inhibitor-1 4G/5G promoter polymorphism and coagulation factor VII Arg353-->Gln polymorphism in Korean patients with coronary artery disease
- Transforming Growth Factor-beta1 Promoter Polymorphism in Childhood Asthma
- Pulmonary Thromboembolism Caused by PROS1 Gene Mutation
- Tumor Necrosis Factor and Lymphotoxin-alpha Gene Polymorphism in Korean Children with Type 1 Diabetes
- A Case of Factor VII Deficiency with Normal Factor VII Antigen Level