J Korean Ophthalmol Soc.
2016 Jul;57(7):1176-1179. 10.3341/jkos.2016.57.7.1176.
Two Cases of External Ophthalmoplegia after Vincristine Treatment in Childhood
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea. jungjaeho@pusan.ac.kr
- KMID: 2317583
- DOI: http://doi.org/10.3341/jkos.2016.57.7.1176
Abstract
- PURPOSE
To report 2 cases of extraocular muscle paresis with ptosis after vincristine treatment in childhood with acute leukemia.
CASE SUMMARY
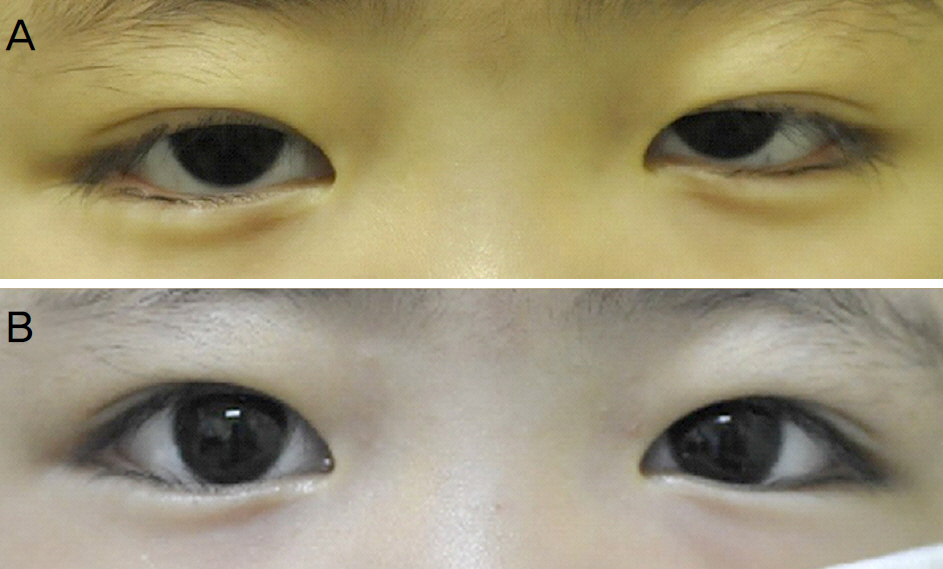
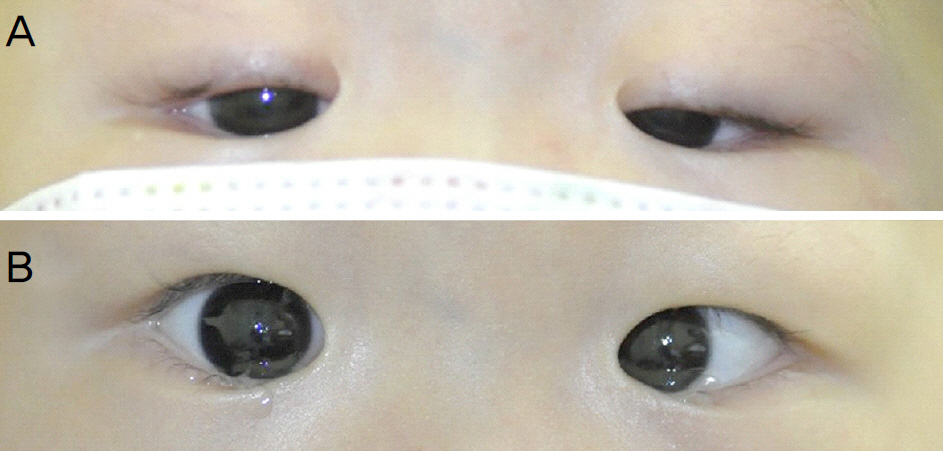
A 4-year-old girl with acute lymphoblastic leukemia experienced diplopia, esotropia and bilateral ptosis after intravenous vincristine treatment. Ptosis improved after two weeks of pyridoxine treatment with vincristine reduction. After 2 months, left abduction defect and ptosis completely disappeared with pyridoxine treatment. A 23-month-old boy with acute lymphoblastic leukemia had bilateral ptosis with worsening pre-existing infantile esotropia after intravenous vincristine treatment. Bilateral ptosis resolved and esotropia improved after vincristine reduction with pyridoxine treatment for 2 months.
CONCLUSIONS
We observed extraocular muscle paresis and ptosis after vincristine treatment for acute leukemia in pediatric patients. Physicians should be suspicious of toxic neuropathy related to vincristine treatment when patients have extraocular ophthalmoplegia, and physicians should know that vincristine reduction with pyridoxine supplement may be helpful.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Rowinsky EK, Donehower RC. The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics. Pharmacol Ther. 1991; 52:35–84.
Article2. Rosenthal S, Kaufman S. Vincristine neurotoxicity. Ann Intern Med. 1974; 80:733–7.
Article3. Sioka C, Kyritsis AP. Central and peripheral nervous system abdominal of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009; 63:761–7.4. Sarkar S, Deb AR, Saha K, Das CS. Simultaneous isolated bilateral facial palsy: a rare vincristine-associated toxicity. Indian J Med Sci. 2009; 63:355–8.5. Gursel O, Sari E, Altun D, et al. Vincristine-induced unilateral abdominal in a child. Pediatr Neurol. 2009; 41:461–3.6. Dejan S, Dragana B, Ivana P, et al. Vincristine induced unilateral ptosis. J Pediatr Hematol Oncol. 2009; 31:463.
Article7. Batta B, Trechot F, Cloché V, et al. Vincristine-induced unilateral ptosis: case report and review of the literature. J Fr Ophtalmol. 2013; 36:683–6.8. Dixit G, Dhingra A, Kaushal D. Vincristine induced cranial neuropathy. J Assoc Physicians India. 2012; 60:56–8.9. Park JM. Abducens nerve palsy induced by chemotherapeutic agents. J Korean Ophthalmol Soc. 2008; 49:1871–6.
Article10. Back SY, Park BB, Park DW, et al. A case of vincristine-induced polyneuropathy in an patient with acute leukemia. Korean J Med. 2009; 76:611–6.11. Scott AB, Kraft SP. Botulinum toxin injection in the management of lateral rectus paresis. Ophthalmology. 1985; 92:676–83.
Article12. Davis RE, Schlumpf BE, Klinger PD. Comparative neurotoxicity of tubulin-binding drugs: inhibition of goldfish optic nerve regeneration. Toxicol Appl Pharmacol. 1985; 80:308–15.
Article13. Green LS, Donoso JA, Heller-Bettinger IE, Samson FE. Axonal transport disturbances in vincristine-induced peripheral neuropathy. Ann Neurol. 1977; 1:255–62.
Article14. Bay A, Yilmaz C, Yilmaz N, Oner AF. Vincristine induced cranial polyneuropathy. Indian J Pediatr. 2006; 73:531–3.
Article15. Chan JD. Pharmacokinetic drug interactions of vinca alkaloids: summary of case reports. Pharmacotherapy. 1998; 18:1304–7.16. Jeng MR, Feusner J. Itraconazole-enhanced vincristine abdominal in a child with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2001; 18:137–42.17. Gomber S, Dewan P, Chhonker D. Vincristine induced abdominal in cancer patients. Indian J Pediatr. 2010; 77:97–100.18. Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012; 291:1–9.
Article19. Müller L, Kramm CM, Tenenbaum T, et al. Treatment of vincris-tine-induced bilateral ptosis with pyridoxine and pyridostigmine. Pediatr Blood Cancer. 2004; 42:287–8.
Article20. Ozyurek H, Turker H, Akbalik M, et al. Pyridoxine and pyridostigmine treatment in vincristine-induced neuropathy. Pediatr Hematol Oncol. 2007; 24:447–52.
Article21. Dalton K, Dalton MJ. Characteristics of pyridoxine overdose abdominal syndrome. Acta Neurol Scand. 1987; 76:8–11.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Internal and External Ophthalmoplegia without Ataxia and Areflexia Associated with Anti-GQ1b Antibody
- Accidental Intrathecal Vincristine Administration
- Analysis of Oculomotor Nerve Palsy due to Internal Carotid-Posterior Communicating Artery Aneurysm
- Vincristine Effect for the Treatment of Idiopathic Thrombocytopenic Purpura in Childhood
- A Case of Miller Fisher Syndrome (Variant of Guillain Barr'e Syndrome-Ophthalmoplegia, Ataxia, Areflexia)