Restor Dent Endod.
2014 Aug;39(3):201-209.
Comparative analysis of physicochemical properties of root perforation sealer materials
- Affiliations
-
- 1Departament of Endodontics, Faculty of Dentistry, University of Cuiaba, Cuiaba, Mato grosso, Brazil. alvarohborges@ gmail.com
- 2Departament of Prosthodontic Dentistry, Faculty of Dentistry, CEUMA University, Sao Luis, Maranhao, Brazil.
- 3Departament of Chemistry, Institute of Exact Sciences and Earth, Mato Grosso Federal University, Cuiaba, Mato Grosso, Brazil.
Abstract
OBJECTIVES
This study evaluated the solubility, dimensional alteration, pH, electrical conductivity, and radiopacity of root perforation sealer materials.
MATERIALS AND METHODS
For the pH test, the samples were immersed in distilled water for different periods of time. Then, the samples were retained in plastic recipients, and the electrical conductivity of the solution was measured. The solubility, dimensional alteration, and radiopacity properties were evaluated according to Specification No. 57 of the American National Standards Institute/American Dental Association (ANSI/ADA). Statistical analyses were carried out using analysis of variance (ANOVA) and Tukey's test at a significance level of 5%. When the sample distribution was not normal, a nonparametric ANOVA was performed with a Kruskal-Wallis test (alpha = 0.05).
RESULTS
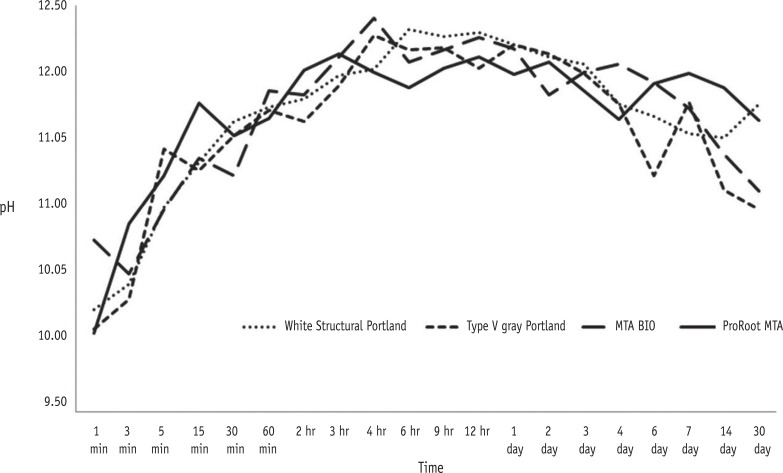
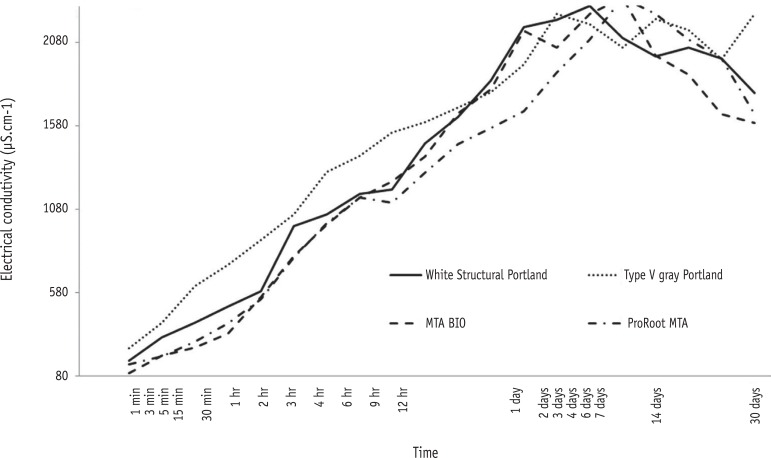
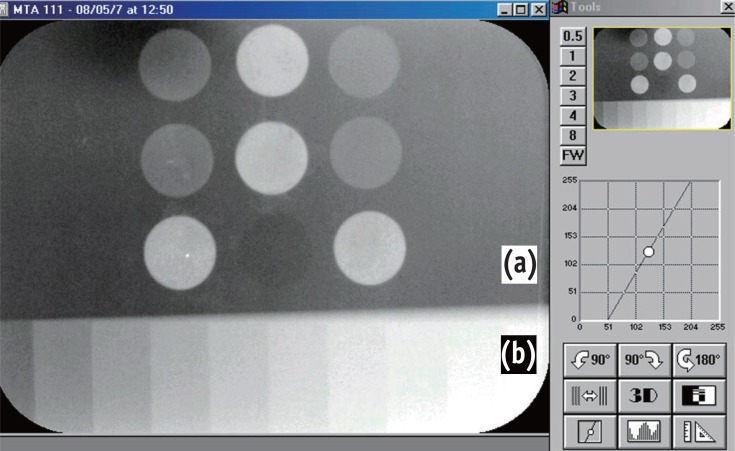
The results showed that white structural Portland cement (PC) had the highest solubility, while mineral trioxide aggregate (MTA)-based cements, ProRoot MTA (Dentsply-Tulsa Dental) and MTA BIO (Angelus Ind. Prod.), had the lowest values. MTA BIO showed the lowest dimensional alteration values and white PC presented the highest values. No differences among the tested materials were observed in the the pH and electrical conductivity analyses. Only the MTA-based cements met the ANSI/ADA recommendations regarding radiopacity, overcoming the three steps of the aluminum step wedge.
CONCLUSIONS
On the basis of these results, we concluded that the values of solubility and dimensional alteration of the materials were in accordance with the ANSI/ADA specifications. PCs did not fulfill the ANSI/ADA requirements regarding radiopacity. No differences were observed among the materials with respect to the pH and electrical conductivity analyses.
MeSH Terms
Figure
Reference
-
1. Krupp C, Bargholz C, Brüsehaber M, Hülsmann M. Treatment outcome after repair of root perforations with mineral trioxide aggregate: a retrospective evaluation of 90 teeth. J Endod. 2013; 39:1364–1368. PMID: 24139255.2. Park DS, Sohn SJ, Oh TS, Yoo HM, Park CJ, Yim SH, Lee YK, Kye SB. An electrochemical study of the sealing ability of three retrofilling materials. J Korean Acad Conserv Dent. 2004; 29:365–369.
Article3. Darvell BW, Wu RC. "MTA"-an Hydraulic Silicate Cement: review update and setting reaction. Dent Mater. 2011; 27:407–422. PMID: 21353694.
Article4. Malhotra N, Agarwal A, Mala K. Mineral trioxide aggregate: a review of physical properties. Compend Contin Educ Dent. 2013; 34:e25–e32. PMID: 23627406.5. Borges AH, Pedro FL, Miranda CE, Semenoff-Segundo A, Pécora JD, Filho AM. Comparative study of physicochemical properties of MTA-based and Portland cements. Acta Odontol Latinoam. 2010; 23:175–181. PMID: 21638956.6. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413. PMID: 20171353.
Article7. Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007; 40:462–470. PMID: 17459120.
Article8. Lee JH, Shon WJ, Lee W, Baek SH. The effect of several root-end filling materials on MG63 osteoblast-like cells. J Korean Acad Conserv Dent. 2010; 35:222–228.
Article9. Saxena P, Gupta SK, Newaskar V. Biocompatibility of root-end filling materials: recent update. Restor Dent Endod. 2013; 38:119–127. PMID: 24010077.
Article10. Song M, Yoon TS, Kim SY, Kim E. Cytotoxicity of newly developed pozzolan cement and other root-end filling materials on human periodontal ligament cell. Restor Dent Endod. 2014; 39:39–44. PMID: 24516828.
Article11. Cavenago BC, Pereira TC, Duarte MA, Ordinola-Zapata R, Marciano MA, Bramante CM, Bernardineli N. Influence of powder-to-water ratio on radiopacity, setting time, pH, calcium ion release and a micro-CT volumetric solubility of white mineral trioxide aggregate. Int Endod J. 2014; 47:120–126. PMID: 23647286.
Article12. Silva Neto JD, Brito RH, Schnaider TB, Gragnani A, Engelman M, Ferreira LM. Root perforations treatment using mineral trioxide aggregate and Portland cements. Acta Cir Bras. 2010; 25:479–484. PMID: 21120277.
Article13. Silva Neto JD, Schnaider TB, Gragnani A, Paiva AP, Novo NF, Ferreira LM. Portland cement with additives in the repair of furcation perforations in dogs. Acta Cir Bras. 2012; 27:809–814. PMID: 23117614.
Article14. Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005; 38:834–842. PMID: 16218977.
Article15. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993; 19:591–595. PMID: 8151252.
Article16. Chang SW. Chemical characteristics of mineral trioxide aggregate and its hydration reaction. Restor Dent Endod. 2012; 37:188–193. PMID: 23429542.
Article17. Estrela C, Bammann LL, Estrela CR, Silva RS, Pécora JD. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J. 2000; 11:3–9. PMID: 11210272.18. Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: a review of the constituents and biological properties of the material. Int Endod J. 2006; 39:747–754. PMID: 16948659.
Article19. ABCP. Basic guide of Portland cement utilization. 7th ed. São Paulo: Brazilian Association of Portland Cement;2002. p. 28.20. Garboczi EJ, Bullard JW. Shape analysis of a reference cement. Cem Concr Res. 2004; 34:1933–1937.
Article21. Borges AH, Pedro FL, Semanoff-Segundo A, Miranda CE, Pécora JD, Cruz Filho AM. Radiopacity evaluation of Portland and MTA-based cements by digital radiographic system. J Appl Oral Sci. 2011; 19:228–232. PMID: 21625738.
Article22. Dammaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005; 21:731–738. PMID: 15935463.
Article23. ANSI/ADA. Specification No. 57. Endodontic sealing materials. Chicago: American Dental Association Publishing;2000.24. Carvalho-Junior JR, Correr-Sobrinho L, Correr AB, Sinhoreti MA, Consani S, Sousa-Neto MD. Solubility and dimensional change after setting of root canal sealers: a proposal for smaller dimensions of test samples. J Endod. 2007; 33:1110–1116. PMID: 17931945.25. Tanomaru-Filho M, da Silva GF, Duarte MA, Gonçalves M, Tanomaru JM. Radiopacity evaluation of root-end filling materials by digitization of images. J Appl Oral Sci. 2008; 16:376–379. PMID: 19082394.
Article26. Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and portland cement. J Endod. 2006; 32:193–197. PMID: 16500224.
Article27. Hwang YC, Lee SH, Hwang IN, Kang IC, Kim MS, Kim SH, Son HH, Oh WM. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:e96–e102. PMID: 19157923.
Article28. Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006; 32:569–572. PMID: 16728254.
Article29. Duarte MA, Alves de Aguiar K, Zeferino MA, Vivan RR, Ordinola-Zapata R, Tanomaru-Filho M, Weckwerth PH, Kuga MC. Evaluation of the propylene glycol association on some physical and chemical properties of mineral trioxide aggregate. Int Endod J. 2012; 45:565–570. PMID: 22720325.
Article30. Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J Endod. 2003; 29:814–817. PMID: 14686812.
Article31. de Martins GR, Carvalho CA, Valera MC, de Oliveira LD, Buso L, Carvalho AS. Sealing ability of castor oil polymer as a root-end filling material. J Appl Oral Sci. 2009; 17:220–223. PMID: 19466255.32. Danesh G, Dammaschke T, Gerth HU, Zandbiglari T, Schäfer E. A comparative study of selected properties of ProRoot mineral trioxide aggregate and two Portland cements. Int Endod J. 2006; 39:213–219. PMID: 16507075.
Article33. Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009; 35:550–554. PMID: 19345803.
Article34. Fridland M, Rosado R. MTA solubility: a long term study. J Endod. 2005; 31:376–379. PMID: 15851933.
Article35. Wiltbank KB, Schwartz SA, Schindler WG. Effect of selected accelerants on the physical properties of mineral trioxide aggregate and Portland cement. J Endod. 2007; 33:1235–1238. PMID: 17889697.
Article36. Duarte MA, Demarchi AC, Yamashita JC, Kuga MC, Fraga Sde C. pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 95:345–347. PMID: 12627108.
Article37. Estrela C, Sousa-Neto MD, Guedes OA, Alencar AH, Duarte MA, Pécora JD. Characterization of calcium oxide in root perforation sealer materials. Braz Dent J. 2012; 23:539–546. PMID: 23306231.
Article38. Santos AD, Moraes JC, Araújo EB, Yukimitu K, Valério Filho WV. Physico-chemical properties of MTA and a novel experimental cement. Int Endod J. 2005; 38:443–447. PMID: 15946264.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vitro evaluation of a newly produced resin-based endodontic sealer
- A scientometric, bibliometric, and thematic map analysis of hydraulic calcium silicate root canal sealers
- The status of clinical trials regarding root canal sealers
- Cytotoxicity of resin-based root canal sealer, adseal
- Micro-computed tomographic evaluation of a new system for root canal filling using calcium silicatebased root canal sealers