Pediatr Gastroenterol Hepatol Nutr.
2015 Jun;18(2):73-84. 10.5223/pghn.2015.18.2.73.
Characteristics of Pediatric Pancreatitis on Magnetic Resonance Cholangiopancreatography
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. hk2005.yoon@gmail.com
- 2Department of Radiology, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 3Department of Radiology, Kangwon National University Hospital, Chuncheon, Korea.
- 4Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2315553
- DOI: http://doi.org/10.5223/pghn.2015.18.2.73
Abstract
- Pediatric pancreatitis is not uncommon and results in considerable morbidity and mortality in the affected children. Unlike adults, pediatric pancreatitis is more frequently associated with underlying structural abnormalities, trauma, and drugs rather than an idiopathic etiology. Magnetic resonance cholangiopancreatography (MRCP) is a good imaging modality for evaluating pancreatitis and determining etiology without exposure to radiation. This article focuses on MRCP findings associated with various causes of pancreatitis in children, particularly structural abnormalities of the pancreaticobiliary system, as well as describing the feasibility, limitations, and solutions associated with pediatric MRCP.
Keyword
MeSH Terms
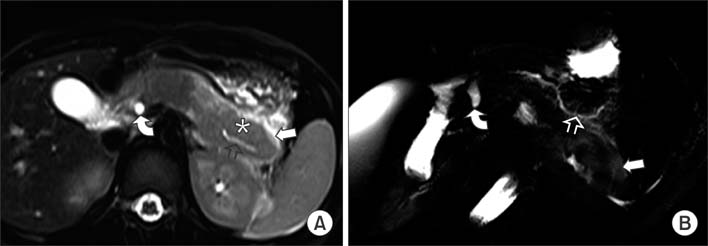
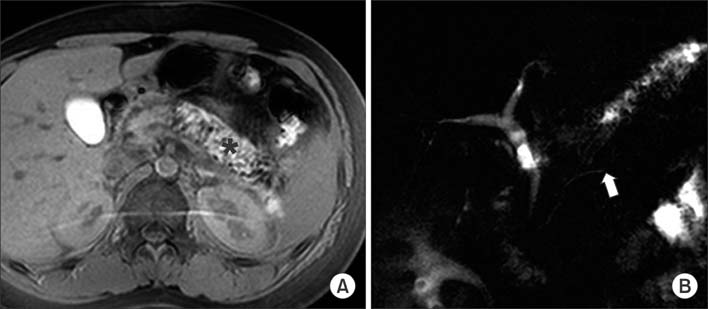
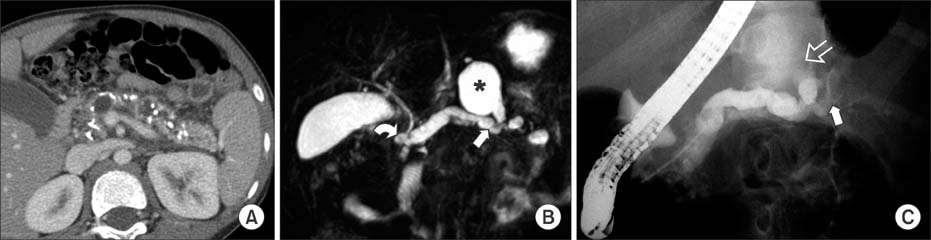
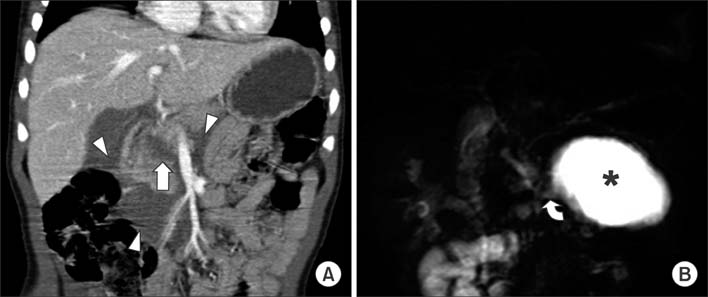
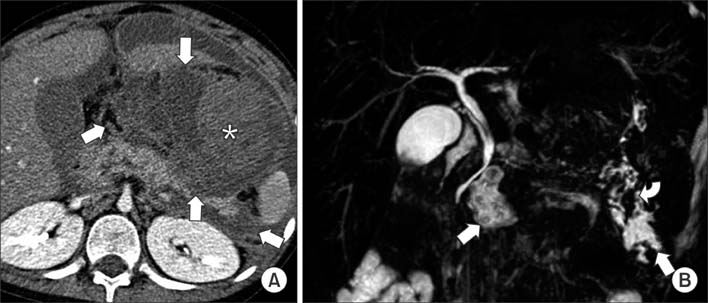
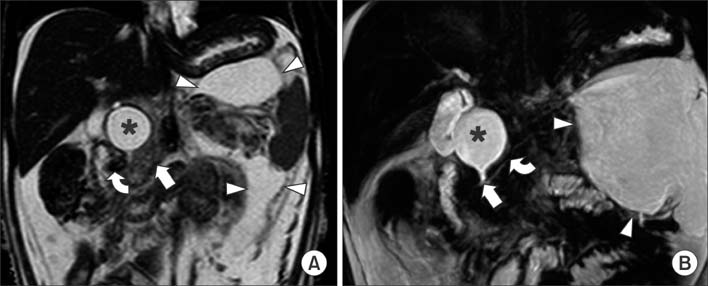
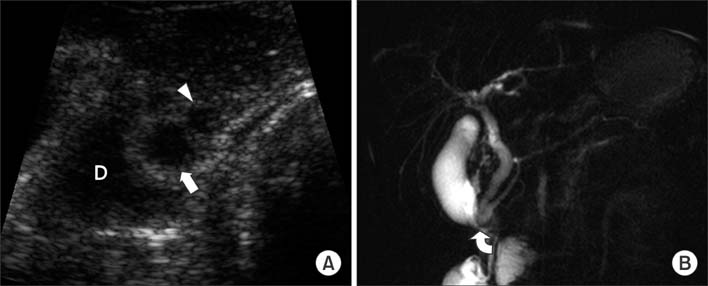
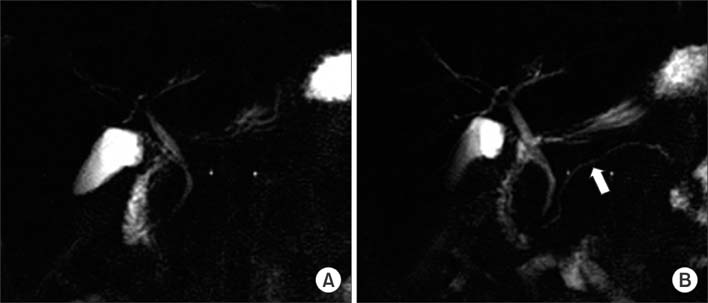
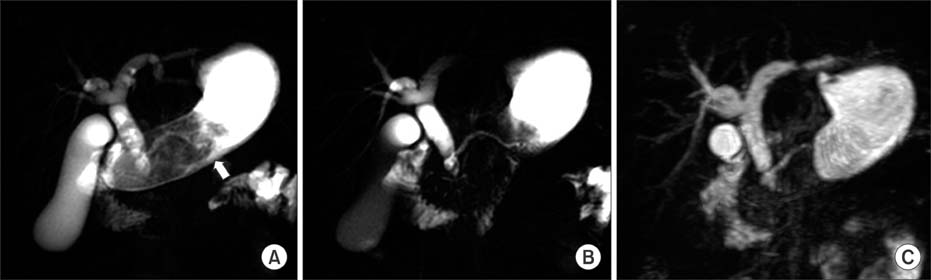
Figure
Reference
-
1. Lopez MJ. The changing incidence of acute pancreatitis in children: a single-institution perspective. J Pediatr. 2002; 140:622–624.
Article2. Nydegger A, Heine RG, Ranuh R, Gegati-Levy R, Crameri J, Oliver MR. Changing incidence of acute pancreatitis: 10-year experience at the Royal Children's Hospital, Melbourne. J Gastroenterol Hepatol. 2007; 22:1313–1316.
Article3. Werlin SL, Kugathasan S, Frautschy BC. Pancreatitis in children. J Pediatr Gastroenterol Nutr. 2003; 37:591–595.
Article4. Benifla M, Weizman Z. Acute pancreatitis in childhood: analysis of literature data. J Clin Gastroenterol. 2003; 37:169–172.5. Choi BH, Lim YJ, Yoon CH, Kim EA, Park YS, Kim KM. Acute pancreatitis associated with biliary disease in children. J Gastroenterol Hepatol. 2003; 18:915–921.
Article6. Su WJ, Chen HL, Lai HS, Ni YH, Chang MH. Pancreaticobiliary anomalies is the leading cause of childhood recurrent pancreatitis. J Formos Med Assoc. 2007; 106:119–125.
Article7. Fitoz S, Erden A, Boruban S. Magnetic resonance cholangiopancreatography of biliary system abnormalities in children. Clin Imaging. 2007; 31:93–101.
Article8. Tipnis NA, Dua KS, Werlin SL. A retrospective assessment of magnetic resonance cholangiopancreatography in children. J Pediatr Gastroenterol Nutr. 2008; 46:59–64.
Article9. Clifton MS, Pelayo JC, Cortes RA, Grethel EJ, Wagner AJ, Lee H, et al. Surgical treatment of childhood recurrent pancreatitis. J Pediatr Surg. 2007; 42:1203–1207.
Article10. Matos C, Cappeliez O, Winant C, Coppens E, Devière J, Metens T. MR imaging of the pancreas: a pictorial tour. Radiographics. 2002; 22:e2.
Article11. Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996; 14:141–145.
Article12. Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000; 25:213–216.
Article13. Shanbhogue AK, Fasih N, Surabhi VR, Doherty GP, Shanbhogue DK, Sethi SK. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics. 2009; 29:1003–1026.
Article14. Lee YJ, Kim KM, Choi JH, Lee BH, Kim GH, Yoo HW. High incidence of PRSS1 and SPINK1 mutations in Korean children with acute recurrent and chronic pancreatitis. J Pediatr Gastroenterol Nutr. 2011; 52:478–481.
Article15. Klöppel G, Detlefsen S, Chari ST, Longnecker DS, Zamboni G. Autoimmune pancreatitis: the clinicopathological characteristics of the subtype with granulocytic epithelial lesions. J Gastroenterol. 2010; 45:787–793.
Article16. Blejter J, Weller S, Pace R, Cusumano H, Giambini D. Autoimmune pancreatitis: an adolescent case and review of literature. J Pediatr Surg. 2008; 43:1368–1372.
Article17. Leva E, Huscher C, Rode H, Fava G, Napolitano M, Maestri L, et al. Management of traumatic complete pancreatic fracture in a child: case report and review of literature. J Laparoendosc Adv Surg Tech A. 2008; 18:321–323.
Article18. Yang L, Zhang XM, Xu XX, Tang W, Xiao B, Zeng NL. MR imaging for blunt pancreatic injury. Eur J Radiol. 2010; 75:e97–e101.
Article19. Balani AR, Grendell JH. Drug-induced pancreatitis: incidence, management and prevention. Drug Saf. 2008; 31:823–837.20. Treepongkaruna S, Thongpak N, Pakakasama S, Pienvichit P, Sirachainan N, Hongeng S. Acute pancreatitis in children with acute lymphoblastic leukemia after chemotherapy. J Pediatr Hematol Oncol. 2009; 31:812–815.
Article21. Kuhn JP, Slovis TL, Haller JO, Caffey J. Caffey's pediatric diagnostic imaging. 10th ed. Philadelphia: Mosby;2004.22. Okada A, Nakamura T, Higaki J, Okumura K, Kamata S, Oguchi Y. Congenital dilatation of the bile duct in 100 instances and its relationship with anomalous junction. Surg Gynecol Obstet. 1990; 171:291–298.23. Suzuki M, Shimizu T, Kudo T, Suzuki R, Otsuka Y, Nagata S, et al. Usefulness of non-breath-hold one shot magnetic resonance cholangiopancreatography for the evaluation of choledochal cyst in children: 189*. J Pediatr Gastroenterol Nutr. 2005; 41:551–552.
Article24. Sonoda M, Sato M, Miyauchi Y, Yazumi S, Nakamura M. A rare case of choledochocele associated with pancreas divisum. Pediatr Surg Int. 2009; 25:991–994.
Article25. Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977; 134:263–269.26. Mori K, Nagakawa T, Ohta T, Nakano T, Kadoya N, Kayahara M, et al. Acute pancreatitis associated with anomalous union of the pancreaticobiliary ductal system. J Clin Gastroenterol. 1991; 13:673–677.
Article27. Guelrud M, Morera C, Rodriguez M, Prados JG, Jaén D. Normal and anomalous pancreaticobiliary union in children and adolescents. Gastrointest Endosc. 1999; 50:189–193.
Article28. Philpott C, Rosenbaum J, Moon A, Bekhit E, Kumbla S. Paediatric MRCP: 10 year experience with 195 patients. Eur J Radiol. 2013; 82:699–706.
Article29. Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc. 2004; 60:419–425.
Article30. Delhaye M, Engelholm L, Cremer M. Pancreas divisum: congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology. 1985; 89:951–958.
Article31. Hayakawa T, Kondo T, Shibata T, Sugimoto Y, Kitagawa M, Suzuki T, et al. Pancreas divisum. A predisposing factor to pancreatitis? Int J Pancreatol. 1989; 5:317–326.32. Delaney L, Applegate KE, Karmazyn B, Akisik MF, Jennings SG. MR cholangiopancreatography in children: feasibility, safety, and initial experience. Pediatr Radiol. 2008; 38:64–75.
Article33. Chavhan GB, Almehdar A, Moineddin R, Gupta S, Babyn PS. Comparison of respiratory-triggered 3-D fast spin-echo and single-shot fast spin-echo radial slab MR cholangiopancreatography images in children. Pediatr Radiol. 2013; 43:1086–1092.
Article34. Irie H, Honda H, Kuroiwa T, Yoshimitsu K, Aibe H, Shinozaki K, et al. Pitfalls in MR cholangiopancreatographic interpretation. Radiographics. 2001; 21:23–37.
Article35. Chavhan GB, Babyn PS, Manson D, Vidarsson L. Pediatric MR cholangiopancreatography: principles, technique, and clinical applications. Radiographics. 2008; 28:1951–1962.
Article36. Dreiling DA, Messer J. The secretin story: a saga in clinical medicine and gastrointestinal physiology. Am J Gastroenterol. 1978; 70:455–479.37. Geenen JE, Hogan WJ, Dodds WJ, Stewart ET, Arndorfer RC. Intraluminal pressure recording from the human sphincter of Oddi. Gastroenterology. 1980; 78:317–324.
Article38. Coppens E, Metens T, Winant C, Matos C. Pineapple juice labeled with gadolinium: a convenient oral contrast for magnetic resonance cholangiopancreatography. Eur Radiol. 2005; 15:2122–2129.
Article39. Ghanaati H, Rokni-Yazdi H, Jalali AH, Abahashemi F, Shakiba M, Firouznia K. Improvement of MR cholangiopancreatography (MRCP) images after black tea consumption. Eur Radiol. 2011; 21:2551–2557.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correction: Characteristics of Pediatric Pancreatitis on Magnetic Resonance Cholangiopancreatography
- Ansa Pancreatica: A Case Report of a Type of Ductal Variation in a Patient with Idiopathic Acute Recurrent Pancreatitis
- Diagnostic Approach to Recurrent Idiopathic Pancreatitis
- Indications and Timing of ERCP and Cholecystectomy for Biliary Pancreatitis
- Non-invasive MR Demonstration of the Fistula between Pancreatic Pseudocyst and Portal Vein: A Case Report