Pediatr Gastroenterol Hepatol Nutr.
2014 Jun;17(2):61-73.
Middle East Consensus Statement on the Prevention, Diagnosis, and Management of Cow's Milk Protein Allergy
- Affiliations
-
- 1Department of Pediatrics, Universitair Ziekenhuis Brussel, Vrije Universiteit Brussel, Brussels, Belgium. yvan.vandenplas@uzbrussels.be
- 2King Abdullah Bin Abdul Aziz University Hospital, Princess Nora Bint Abdulrahman University, Riyadh, Kingdom of Saudi Arabia.
- 3Department of Pediatrics, College of Medicine and Health Sciences, United Arab Emirates University, Al-Ain, United Arab Emirates.
- 4Department of Child Health and Nutrition, Institute of Postgraduate Childhood Studies, Ain Shams University, Cairo, Egypt.
- 5Division of Pediatric Gastroenterology, Hepatology & Nutrition, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates.
- 6Department of Pediatrics, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
- 7Department of Pediatrics, Hotel-Dieu de France, St. Joseph University, Beirut, Lebanon.
Abstract
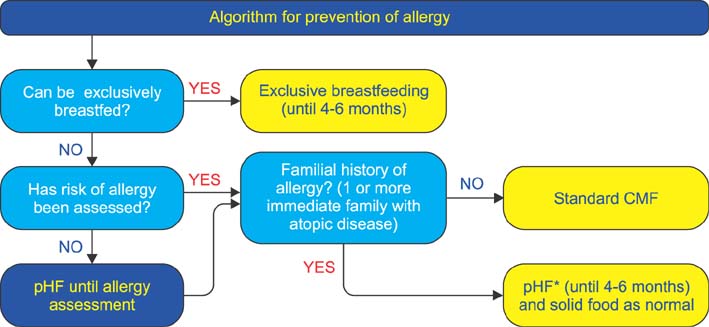
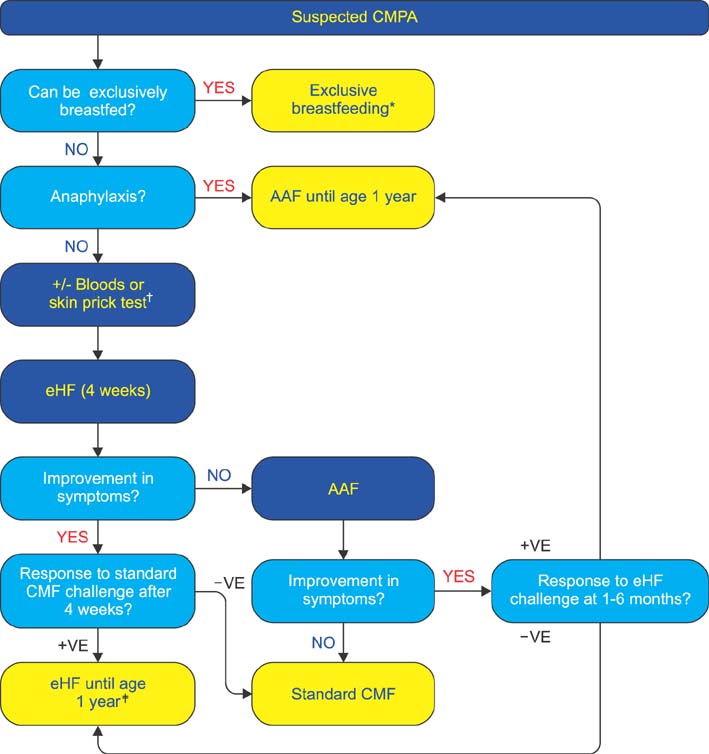
- Presented are guidelines for the prevention, diagnosis, and treatment of cow's milk protein allergy (CMPA) which is the most common food allergy in infants. It manifests through a variety of symptoms that place a burden on both the infant and their caregivers. The guidelines were formulated by evaluation of existing evidence-based guidelines, literature evidence and expert clinical experience. The guidelines set out practical recommendations and include algorithms for the prevention and treatment of CMPA. For infants at risk of allergy, appropriate prevention diets are suggested. Breastfeeding is the best method for prevention; however, a partially hydrolyzed formula should be used in infants unable to be breastfed. In infants with suspected CMPA, guidelines are presented for the appropriate diagnostic workup and subsequent appropriate elimination diet for treatment. Exclusive breastfeeding and maternal dietary allergen avoidance are the best treatment. In infants not exclusively breastfed, an extensively hydrolyzed formula should be used with amino acid formula recommended if the symptoms are life-threatening or do not resolve after extensively hydrolyzed formula. Adherence to these guidelines should assist healthcare practitioners in optimizing their approach to the management of CMPA and decrease the burden on infants and their caregivers.
Keyword
MeSH Terms
Figure
Reference
-
1. Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, Beyer K, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines. World Allergy Organ J. 2010; 3:57–161.
Article2. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. NIAID-Sponsored Expert Panel. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010; 126:1105–1118.
Article3. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011; 127:594–602.
Article4. Pawankar R, Canonica GW, Holgate ST, Lockey RF. WAO white book on allergy 2011-2012: executive summary. Milwaukee: World Allergy Organization;2011.5. Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006; 117:1118–1124.
Article6. Høst A. Frequency of cow's milk allergy in childhood. Ann Allergy Asthma Immunol. 2002; 89:6 Suppl 1. 33–37.
Article7. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012; 55:221–229.
Article8. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000; 106:346–349.9. Feijen M, Gerritsen J, Postma DS. Genetics of allergic disease. Br Med Bull. 2000; 56:894–907.
Article10. Greer FR, Sicherer SH, Burks AW. American Academy of Pediatrics Committee on Nutrition. American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008; 121:183–191.
Article11. Høst A, Halken S, Muraro A, Dreborg S, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Pediatr Allergy Immunol. 2008; 19:1–4.12. Aberg N, Engström I, Lindberg U. Allergic diseases in Swedish school children. Acta Paediatr Scand. 1989; 78:246–252.
Article13. Kjellman NI. Atopic disease in seven-year-old children. Incidence in relation to family history. Acta Paediatr Scand. 1977; 66:465–471.
Article14. American College of Allergy, Asthma, & Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006; 96:3 Suppl 2. S1–S68.15. von Berg A, Filipiak-Pittroff B, Krämer U, Hoffmann B, Link E, Beckmann C, et al. GINIplus study group. Allergies in high-risk schoolchildren after early intervention with cow's milk protein hydrolysates: 10-year results from the German Infant Nutritional Intervention (GINI) study. J Allergy Clin Immunol. 2013; 131:1565–1573.
Article16. Yang YW, Tsai CL, Lu CY. Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. Br J Dermatol. 2009; 161:373–383.
Article17. Fälth-Magnusson K, Kjellman NI. Allergy prevention by maternal elimination diet during late pregnancy--a 5-year follow-up of a randomized study. J Allergy Clin Immunol. 1992; 89:709–713.
Article18. Lilja G, Dannaeus A, Foucard T, Graff-Lonnevig V, Johansson SG, Oman H. Effects of maternal diet during late pregnancy and lactation on the development of IgE and egg- and milk-specific IgE and IgG antibodies in infants. Clin Exp Allergy. 1991; 21:195–202.
Article19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2006; (3):CD000133.
Article20. Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years' follow-up. Acta Paediatr Suppl. 1996; 414:1–21.
Article21. Vandenplas Y, Hauser B, Van den Borre C, Clybouw C, Mahler T, Hachimi-Idrissi S, et al. The long-term effect of a partial whey hydrolysate formula on the prophylaxis of atopic disease. Eur J Pediatr. 1995; 154:488–494.
Article22. Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatr Allergy Immunol. 2000; 11:149–161.
Article23. de Seta L, Siani P, Cirillo G, Di Gruttola M, Cimaduomo L, Coletta S. The prevention of allergic diseases with a hypoallergenic formula: a follow-up at 24 months. The preliminary results. Pediatr Med Chir. 1994; 16:251–254.24. Osborn DA, Sinn J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. 2006; (4):CD003664.
Article25. Crittenden RG, Bennett LE. Cow's milk allergy: a complex disorder. J Am Coll Nutr. 2005; 24:6 Suppl. 582S–591S.
Article26. Su J, Prescott S, Sinn J, Tang M, Smith P, Heine RG, et al. Cost-effectiveness of partially-hydrolyzed formula for prevention of atopic dermatitis in Australia. J Med Econ. 2012; 15:1064–1077.
Article27. Pedrosa M, Pascual CY, Larco JI, Esteban MM. Palatability of hydrolysates and Other substitution formulas for cow's milk-allergic children: a comparative study of taste, smell, and texture evaluated by healthy volunteers. J Investig Allergol Clin Immunol. 2006; 16:351–356.28. Berni Canani R, Nocerino R, Terrin G, Frediani T, Lucarelli S, Cosenza L, et al. Formula selection for management of children with cow's milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013; 163:771–777.e1.
Article29. Berni Canani R, Nocerino R, Leone L, Di Costanzo M, Terrin G, Passariello A, et al. Tolerance to a new free amino acid-based formula in children with IgE or non-IgE-mediated cow's milk allergy: a randomized controlled clinical trial. BMC Pediatr. 2013; 13:24.
Article30. Fergusson DM, Horwood LJ, Shannon FT. Early solid feeding and recurrent childhood eczema: a 10-year longitudinal study. Pediatrics. 1990; 86:541–546.
Article31. Morgan J, Williams P, Norris F, Williams CM, Larkin M, Hampton S. Eczema and early solid feeding in preterm infants. Arch Dis Child. 2004; 89:309–314.
Article32. Zutavern A, Brockow I, Schaaf B, Bolte G, von Berg A, Diez U, et al. LISA Study Group. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: results from a prospective birth cohort study. Pediatrics. 2006; 117:401–411.
Article33. Poole JA, Barriga K, Leung DY, Hoffman M, Eisenbarth GS, Rewers M, et al. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics. 2006; 117:2175–2182.
Article34. Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008; 122:984–991.
Article35. Snijders BE, Thijs C, van Ree R, van den Brandt PA. Age at first introduction of cow milk products and other food products in relation to infant atopic manifestations in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2008; 122:e115–e122.
Article36. Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010; 126:807–813.
Article37. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. 2013; 3:CD006474.
Article38. Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003; 361:1869–1871.
Article39. Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008; 138:1091–1095.
Article40. Vandenplas Y, Koletzko S, Isolauri E, Hill D, Oranje AP, Brueton M, et al. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Arch Dis Child. 2007; 92:902–908.
Article41. De Greef E, Hauser B, Devreker T, Veereman-Wauters G, Vandenplas Y. Diagnosis and management of cow's milk protein allergy in infants. World J Pediatr. 2012; 8:19–24.
Article42. Costa AJ, Sarinho ES, Motta ME, Gomes PN, de Oliveira de Melo SM, da Silva GA. Allergy to cow's milk proteins: what contribution does hypersensitivity in skin tests have to this diagnosis? Pediatr Allergy Immunol. 2011; 22:e133–e138.
Article43. Celik-Bilgili S, Mehl A, Verstege A, Staden U, Nocon M, Beyer K, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005; 35:268–273.
Article44. Verstege A, Mehl A, Rolinck-Werninghaus C, Staden U, Nocon M, Beyer K, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy. 2005; 35:1220–1226.
Article45. Giampietro PG, Kjellman NI, Oldaeus G, Wouters-Wesseling W, Businco L. Hypoallergenicity of an extensively hydrolyzed whey formula. Pediatr Allergy Immunol. 2001; 12:83–86.
Article46. Hill DJ, Murch SH, Rafferty K, Wallis P, Green CJ. The efficacy of amino acid-based formulas in relieving the symptoms of cow's milk allergy: a systematic review. Clin Exp Allergy. 2007; 37:808–822.
Article47. Taylor RR, Sladkevicius E, Panca M, Lack G, Guest JF. Cost-effectiveness of using an extensively hydrolysed formula compared to an amino acid formula as first-line treatment for cow milk allergy in the UK. Pediatr Allergy Immunol. 2012; 23:240–249.
Article48. de Boissieu D, Dupont C. Allergy to extensively hydrolyzed cow's milk proteins in infants: safety and duration of amino acid-based formula. J Pediatr. 2002; 141:271–273.
Article49. Järvinen KM, Chatchatee P. Mammalian milk allergy: clinical suspicion, cross-reactivities and diagnosis. Curr Opin Allergy Clin Immunol. 2009; 9:251–258.
Article50. Ehlayel M, Bener A, Abu Hazeima K, Al-Mesaifri F. Camel milk is a safer choice than goat milk for feeding children with cow milk allergy. ISRN Allergy. 2011; 2011:391641.
Article51. Halpern SR, Sellars WA, Johnson RB, Anderson DW, Saperstein S, Reisch JS. Development of childhood allergy in infants fed breast, soy, or cow milk. J Allergy Clin Immunol. 1973; 51:139–151.
Article52. Bhatia J, Greer F. American Academy of Pediatrics Committee on Nutrition. Use of soy protein-based formulas in infant feeding. Pediatrics. 2008; 121:1062–1068.
Article53. Katz Y, Gutierrez-Castrellon P, González MG, Rivas R, Lee BW, Alarcon P. A comprehensive review of sensitization and allergy to soy-based products. Clin Rev Allergy Immunol. 2014; 46:272–281.
Article54. Vandenplas Y, Castrellon PG, Rivas R, Gutiérrez CJ, Garcia LD, Jimenez JE, et al. Safety of soya-based infant formulas in children. Br J Nutr. 2014; 111:1340–1360.
Article55. ESPGHAN Committee on Nutrition. Agostoni C, Axelsson I, Goulet O, Koletzko B, Michaelsen KF, et al. Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2006; 42:352–361.56. D'Auria E, Sala M, Lodi F, Radaelli G, Riva E, Giovannini M. Nutritional value of a rice-hydrolysate formula in infants with cows' milk protein allergy: a randomized pilot study. J Int Med Res. 2003; 31:215–222.57. Vandenplas Y, De Greef E, Hauser B. Paradice Study Group. Faltering weight gain normalizes with an extensively hydrolyzed rice protein formula in the treatment of cow's milk protein allergic infants. Eur J Pediatr. 2014; [Epub ahead of print].58. Jackson BP, Taylor VF, Punshon T, Cottingham KL. Arsenic concentration and speciation in infant formulas and first foods. Pure Appl Chem. 2012; 84:215–223.
Article59. Ludman S, Shah N, Fox AT. Managing cows' milk allergy in children. BMJ. 2013; 347:f5424.
Article60. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2007; 120:1172–1177.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correction: Middle East Consensus Statement on the Prevention, Diagnosis, and Management of Cow's Milk Protein Allergy
- Cow mild allergy in infant who neonatal onset
- A Case of Cow's Milk Allergy with Atopic Dermatitis
- The management of food allergy in Indonesia
- Regional differences in diagnosis and management of cow's milk allergy