Nutr Res Pract.
2015 Apr;9(2):137-143. 10.4162/nrp.2015.9.2.137.
Effects of quercetin derivatives from mulberry leaves: Improved gene expression related hepatic lipid and glucose metabolism in short-term high-fat fed mice
- Affiliations
-
- 1Department of Environmental and Preventive Medicine, Shimane University School of Medicine, 89-1 Enya-cho, Izumo City, Shimane 693-8501, Japan. myamasak@med.shimane-u.ac.jp
- 2Shimane Institute for Industrial Technology, Matsue City, Shimane 690-0816, Japan.
- KMID: 2313821
- DOI: http://doi.org/10.4162/nrp.2015.9.2.137
Abstract
- BACKGROUND/OBJECTIVES
Mulberry leaves contain quercetin derivatives, which have the effects of reducing obesity and improving lipid and glucose metabolism in mice with obesity. It is not clear whether or not mulberry leaves can directly affect metabolic disorders, in the presence of obesity, because of the interaction between obesity and metabolic disorders. The aim of the current study was to assess the direct action of quercetin derivatives on metabolic disorders in non-obese conditions in short-term high-fat diet fed mice.
MATERIALS/METHODS
C57BL/6N mice were fed a high-fat diet, supplemented with either 0% (control), 1%, or 3% mulberry leaf powder (Mul) or 1% catechin powder for five days. Anthropometric parameters and blood biochemistry were determined, and hepatic gene expression associated with lipid and glucose metabolism was analyzed.
RESULTS
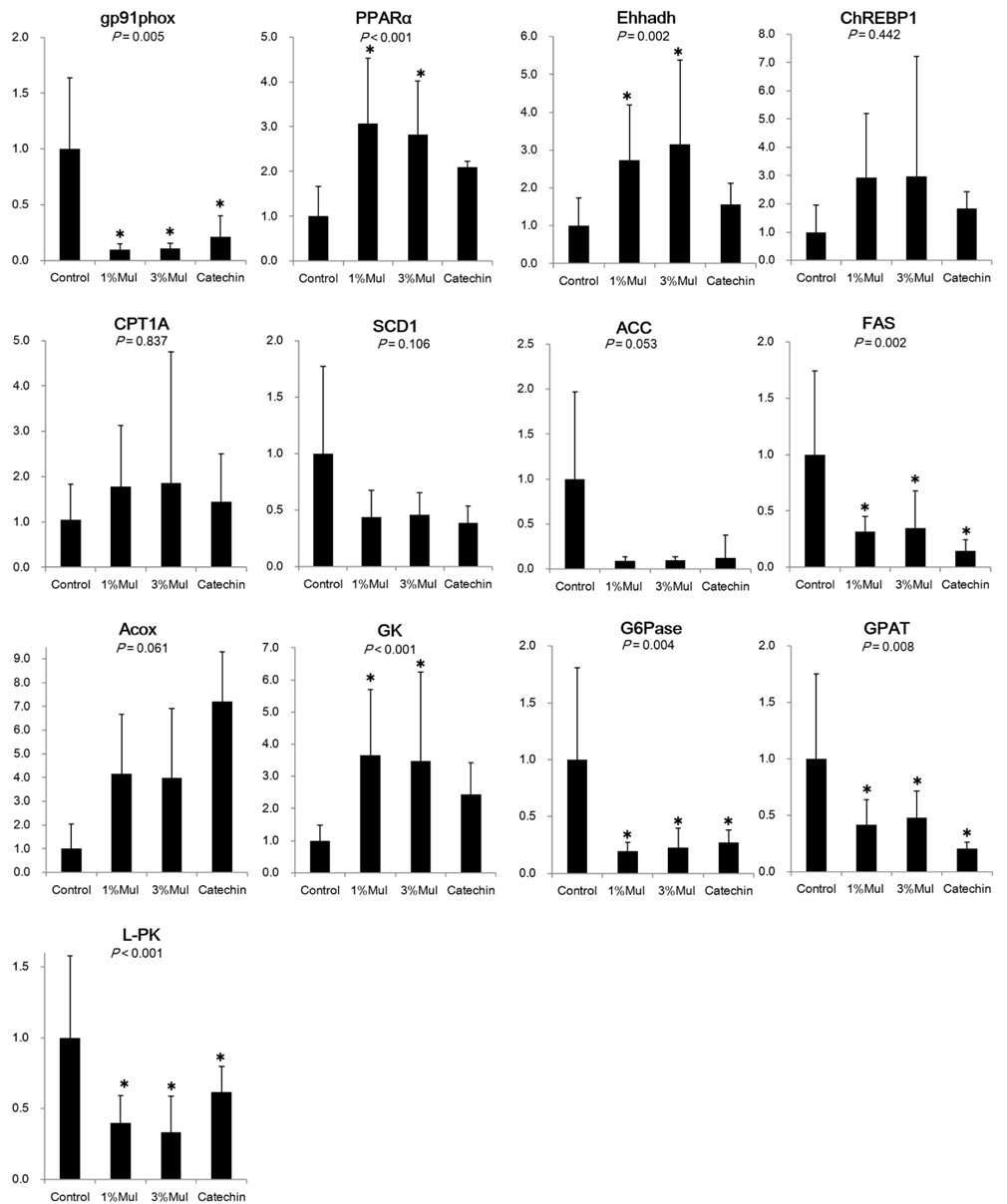
Body and white fat weights did not differ among the four groups. Plasma triglycerides, total cholesterol, and free fatty acids in the 1%, 3% Mul and catechin groups did not differ significantly from those of the controls, however, plasma glucose and 8-isoprostane levels were significantly reduced. Liver gene expression of gp91phox, a main component of NADPH oxidase, was significantly down-regulated, and PPAR-alpha, related to beta-oxidation, was significantly up-regulated. FAS and GPAT, involved in lipid metabolism, were significantly down-regulated, and Ehhadh was significantly up-regulated. Glucose-metabolism related genes, L-PK and G6Pase, were significantly down-regulated, while GK was significantly up-regulated in the two Mul groups compared to the control group.
CONCLUSIONS
Our results suggest that the Mul quercetin derivatives can directly improve lipid and glucose metabolism by reducing oxidative stress and enhancing beta-oxidation. The 1% Mul and 1% catechin groups had similar levels of polyphenol compound intake (0.4 x 10(-5) vs 0.4 x 10(-5) mole/5 days) and exhibited similar effects, but neither showed dose-dependent effects on lipid and glucose metabolism or oxidative stress.
MeSH Terms
-
Adipose Tissue, White
Animals
Biochemistry
Blood Glucose
Catechin
Cholesterol
Diet, High-Fat
Fatty Acids, Nonesterified
Gene Expression*
Glucose*
Lipid Metabolism
Liver
Metabolism*
Mice*
Morus*
NADPH Oxidase
Obesity
Oxidative Stress
Plasma
Quercetin*
Triglycerides
Weights and Measures
Catechin
Cholesterol
Fatty Acids, Nonesterified
Glucose
NADPH Oxidase
Quercetin
Triglycerides
Figure
Reference
-
1. Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002; 76:560–568.
Article2. Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008; 138:1677–1683.
Article3. Katsube T, Imawaka N, Kawano Y, Yamazaki Y, Shiwaku K, Yamane Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. Food Chem. 2006; 97:25–31.
Article4. Wang L, Yamasaki M, Katsube T, Sun X, Yamasaki Y, Shiwaku K. Antiobesity effect of polyphenolic compounds from molokheiya (Corchorus olitorius L.) leaves in LDL receptor-deficient mice. Eur J Nutr. 2011; 50:127–133.
Article5. Enkhmaa B, Shiwaku K, Katsube T, Kitajima K, Anuurad E, Yamasaki M, Yamane Y. Mulberry (Morus alba L.) leaves and their major flavonol quercetin 3-(6-malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor-deficient mice. J Nutr. 2005; 135:729–734.
Article6. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–1761.
Article7. West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000; 17:171–180.
Article8. Thielecke F, Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome - a review. Phytochemistry. 2009; 70:11–24.
Article9. Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord. 2002; 26:1459–1464.
Article10. Feillet-Coudray C, Sutra T, Fouret G, Ramos J, Wrutniak-Cabello C, Cabello G, Cristol JP, Coudray C. Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of polyphenols: Involvement of mitochondrial and NAD(P)H oxidase systems. Free Radic Biol Med. 2009; 46:624–632.11. Kuda T, Iwai A, Yano T. Effect of red pepper Capsicum annuum var. conoides and garlic Allium sativum on plasma lipid levels and cecal microflora in mice fed beef tallow. Food Chem Toxicol. 2004; 42:1695–1700.
Article12. Han LK, Sumiyoshi M, Zhang J, Liu MX, Zhang XF, Zheng YN, Okuda H, Kimura Y. Anti-obesity action of Salix matsudana leaves (Part 1). Anti-obesity action by polyphenols of Salix matsudana in high fat-diet treated rodent animals. Phytother Res. 2003; 17:1188–1194.
Article13. Aoki F, Honda S, Kishida H, Kitano M, Arai N, Tanaka H, Yokota S, Nakagawa K, Asakura T, Nakai Y, Mae T. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci Biotechnol Biochem. 2007; 71:206–214.
Article14. Nemali MR, Usuda N, Reddy MK, Oyasu K, Hashimoto T, Osumi T, Rao MS, Reddy JK. Comparison of constitutive and inducible levels of expression of peroxisomal beta-oxidation and catalase genes in liver and extrahepatic tissues of rat. Cancer Res. 1988; 48:5316–5324.15. Hashimoto T, Fujita T, Usuda N, Cook W, Qi C, Peters JM, Gonzalez FJ, Yeldandi AV, Rao MS, Reddy JK. Peroxisomal and mitochondrial fatty acid beta-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor alpha and peroxisomal fatty acyl-CoA oxidase. Genotype correlation with fatty liver phenotype. J Biol Chem. 1999; 274:19228–19236.
Article16. Shimoda H, Tanaka J, Kikuchi M, Fukuda T, Ito H, Hatano T, Yoshida T. Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. J Agric Food Chem. 2009; 57:1786–1792.
Article17. Ide T, Ashakumary L, Takahashi Y, Kushiro M, Fukuda N, Sugano M. Sesamin, a sesame lignan, decreases fatty acid synthesis in rat liver accompanying the down-regulation of sterol regulatory element binding protein-1. Biochim Biophys Acta. 2001; 1534:1–13.
Article18. Kajikawa S, Harada T, Kawashima A, Imada K, Mizuguchi K. Highly purified eicosapentaenoic acid prevents the progression of hepatic steatosis by repressing monounsaturated fatty acid synthesis in high-fat/high-sucrose diet-fed mice. Prostaglandins Leukot Essent Fatty Acids. 2009; 80:229–238.
Article19. Honda S, Aoki F, Tanaka H, Kishida H, Nishiyama T, Okada S, Matsumoto I, Abe K, Mae T. Effects of ingested turmeric oleoresin on glucose and lipid metabolisms in obese diabetic mice: a DNA microarray study. J Agric Food Chem. 2006; 54:9055–9062.
Article20. Yamazaki T, Kishimoto K, Miura S, Ezaki O. Dietary beta-conglycinin prevents fatty liver induced by a high-fat diet by a decrease in peroxisome proliferator-activated receptor gamma2 protein. J Nutr Biochem. 2012; 23:123–132.
Article21. Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006; 55:928–934.
Article22. Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green teapolyphenol EGCG. Nat Struct Mol Biol. 2004; 11:380–381.23. Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011; 22:1–7.
Article24. Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004; 134:3431S–3440S.
Article25. Ruijters EJ, Weseler AR, Kicken C, Haenen GR, Bast A. The flavanol (-)-epicatechin and its metabolites protect against oxidative stress in primary endothelial cells via a direct antioxidant effect. Eur J Pharmacol. 2013; 715:147–153.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of quercetin on the improvement of lipid metabolism through regulating hepatic AMPK and microRNA-21 in high cholesterol diet-fed mice
- Inhibition of Serotonin Synthesis Induces Negative Hepatic Lipid Balance
- Antioxidant Effects and Improvement of Lipid Metabolism of Mulberry fruit, Mulberry Leaves and Silkworm Powder with Different Mixing Ratios in Streptozotocin-Induced Diabetic Rats
- Anti-obesity effects of hot water extract from Wasabi (Wasabia japonica Matsum.) leaves in mice fed high-fat diets
- Effects of Genistein Supplementation on Fatty Liver and Lipid Metabolism in Rats Fed High Fat Diet