Nutr Res Pract.
2014 Aug;8(4):391-397.
Apple pectin, a dietary fiber, ameliorates myocardial injury by inhibiting apoptosis in a rat model of ischemia/reperfusion
- Affiliations
-
- 1Department of Biochemistry, School of Medicine, Catholic University of Daegu, 33, 17-gil, Duryugongwon-ro, Nam-gu, Daegu 705-718, Korea. leejw@cu.ac.kr
Abstract
- BACKGROUND
/OBJECTIVE: Myocardial cell death due to occlusion of the coronary arteries leads to myocardial infarction, a subset of coronary heart disease (CHD). Dietary fiber is known to be associated with a reduced risk of CHD, the underlying mechanisms of which were suggested to delay the onset of occlusion by ameliorating risk factors. In this study, we tested a hypothesis that a beneficial role of dietary fiber could arise from protection of myocardial cells against ischemic injury, manifested after occlusion of the arteries.
MATERIALS/METHODS
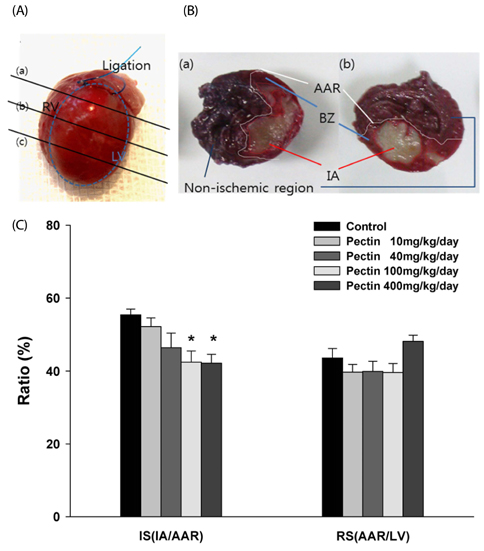
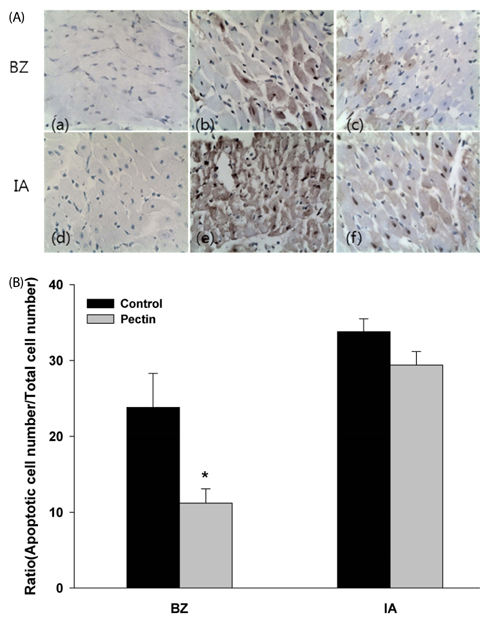
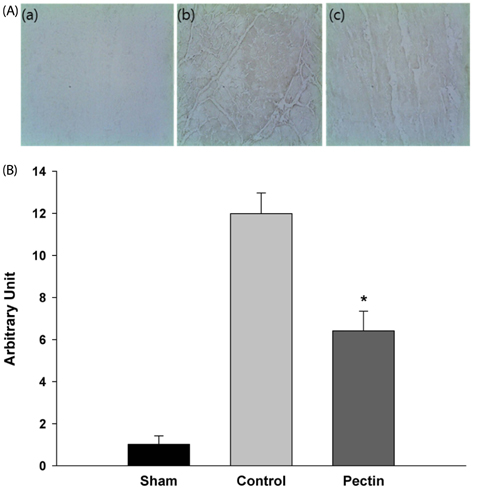
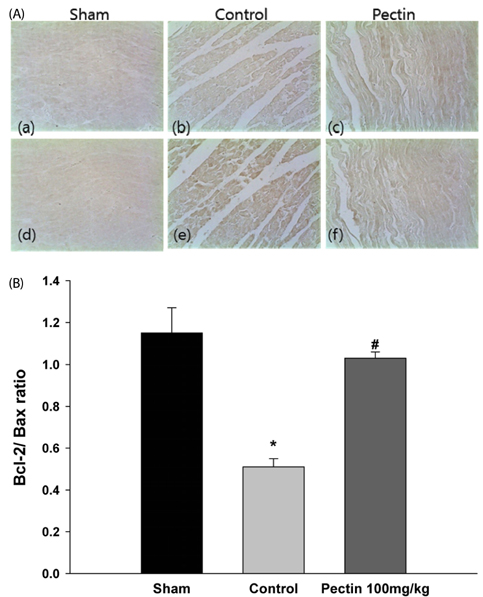
Three days after rats were fed apple pectin (AP) (with 10, 40, 100, and 400 mg/kg/day), myocardial ischemic injury was induced by 30 min-ligation of the left anterior descending coronary artery, followed by 3 hr-reperfusion. The area at risk and infarct area were evaluated using Evans blue dye and 2,3,5-triphenyltetrazolium chloride (TTC) staining, respectively. DNA nicks reflecting the extent of myocardial apoptosis were assessed by TUNEL assay. Levels of cleaved caspase-3, Bcl-2, and Bax were assessed by immunohistochemistry.
RESULTS
Supplementation of AP (with 100 and 400 mg/kg/day) resulted in significantly attenuated infarct size (IS) (ratio of infarct area to area at risk) by 21.9 and 22.4%, respectively, in the AP-treated group, compared with that in the control group. This attenuation in IS showed correlation with improvement in biomarkers involved in the apoptotic cascades: reduction of apoptotic cells, inhibition of conversion of procaspase-3 to caspase-3, and increase of Bcl-2/Bax ratio, a determinant of cell fate.
CONCLUSIONS
The findings indicate that supplementation of AP results in amelioration of myocardial infarction by inhibition of apoptosis. Thus, the current study suggests that intake of dietary fiber reduces the risk of CHD, not only by blocking steps leading to occlusion, but also by protecting against ischemic injury caused by occlusion of the arteries.
Keyword
MeSH Terms
Figure
Reference
-
1. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007; 116:2634–2653.
Article2. Badimon L, Padró T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care. 2012; 1:60–74.
Article3. Chapman MJ. From pathophysiology to targeted therapy for atherothrombosis: a role for the combination of statin and aspirin in secondary prevention. Pharmacol Ther. 2007; 113:184–196.
Article4. Brener SJ. Insights into the pathophysiology of ST-elevation myocardial infarction. Am Heart J. 2006; 151:S4–S10.
Article5. Eshak ES, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Wakai K, Tamakoshi A. JACC Study Group. Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J Nutr. 2010; 140:1445–1453.
Article6. Pereira MA, O'Reilly E, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med. 2004; 164:370–376.7. Bazzano LA, He J, Ogden LG, Loria CM, Whelton PK. National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Dietary fiber intake and reduced risk of coronary heart disease in US men and women: the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Intern Med. 2003; 163:1897–1904.
Article8. Shin D. Analysis of dietary insoluble and soluble fiber contents in school meal. Nutr Res Pract. 2012; 6:28–34.
Article9. Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2008; 108:1716–1731.
Article10. Bell SJ. A review of dietary fiber and health: focus on raisins. J Med Food. 2011; 14:877–883.
Article11. Han HS, Jang JH, Jang JH, Choi JS, Kim YJ, Lee C, Lim SH, Lee HK, Lee J. Water extract of Triticum aestivum L. and its components demonstrate protective effect in a model of vascular dementia. J Med Food. 2010; 13:572–578.
Article12. Hansen L, Dragsted LO, Olsen A, Christensen J, Tjønneland A, Schmidt EB, Overvad K. Fruit and vegetable intake and risk of acute coronary syndrome. Br J Nutr. 2010; 104:248–255.
Article13. Jensen EN, Buch-Andersen T, Ravn-Haren G, Dragsted LO. Mini-review: The effects of apples on plasma cholesterol levels and cardiovascular risk - a review of the evidence. J Hortic Sci Biotechnol. 2009; 84:34–41.
Article14. Brouns F, Theuwissen E, Adam A, Bell M, Berger A, Mensink RP. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur J Clin Nutr. 2012; 66:591–599.
Article15. Sánchez D, Muguerza B, Moulay L, Hernández R, Miguel M, Aleixandre A. Highly methoxylated pectin improves insulin resistance and other cardiometabolic risk factors in Zucker fatty rats. J Agric Food Chem. 2008; 56:3574–3581.
Article16. Nishimura N, Tanabe H, Sasaki Y, Makita Y, Ohata M, Yokoyama S, Asano M, Yamamoto T, Kiriyama S. Pectin and high-amylose maize starch increase caecal hydrogen production and relieve hepatic ischaemia-reperfusion injury in rats. Br J Nutr. 2012; 107:485–492.
Article17. Melton LD, Smith BG. Determination of neutral sugars by gas chromatography of their alditol acetates. Curr Protoc Food Analyt Chem. 2001; E3.2.1–E3.2.13.
Article18. Lim SH, Song KS, Lee JW. Butyrate and propionate, short chain fatty acids, attenuate myocardial damages by inhibition of apoptosis in a rat model of ischemia-reperfusion. J Korean Soc Appl Biol Chem. 2010; 53:570–577.
Article19. Lim SH, Lee J. Methanol extract of Cassia mimosoides var. nomame attenuates myocardial injury by inhibition of apoptosis in a rat model of ischemia-reperfusion. Prev Nutr Food Sci. 2012; 17:177–183.
Article20. Ren J, Babcock SA, Li Q, Huff AF, Li SY, Doser TA. Aldehyde dehydrogenase-2 transgene ameliorates chronic alcohol ingestion-induced apoptosis in cerebral cortex. Toxicol Lett. 2009; 187:149–156.
Article21. Fraeye I, Duvetter T, Verlent I, Sila DN, Hendrickx M, Van Loey A. Comparison of enzymatic de-esterification of strawberry and apple pectin at elevated pressure by fungal pectinmethylesterase. Innov Food Sci Emerg Technol. 2007; 8:93–101.
Article22. Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation [corrected]. Proc Natl Acad Sci U S A. 2004; 101:11471–11476.
Article23. Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005; 11:305–311.
Article24. Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005; 11:1096–1103.
Article25. Krijnen PA, Nijmeijer R, Meijer CJ, Visser CA, Hack CE, Niessen HW. Apoptosis in myocardial ischaemia and infarction. J Clin Pathol. 2002; 55:801–811.
Article26. Zidar N, Dolenc-Strazar Z, Jeruc J, Stajer D. Immunohistochemical expression of activated caspase-3 in human myocardial infarction. Virchows Arch. 2006; 448:75–79.
Article27. Rabkin SW. Apoptosis in human acute myocardial infarction: the rationale for clinical trials of apoptosis inhibition in acute myocardial infarction. Sch Res Exch. 2009; 979318.
Article28. Hengartner MO. The biochemistry of apoptosis. Nature. 2000; 407:770–776.
Article29. Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005; 310:66–67.
Article30. Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006; 311:847–851.
Article31. Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001; 26:61–66.
Article32. van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res. 2005; 67:21–29.
Article33. Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009; 14:536–548.
Article34. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–619.
Article35. Abbate A, Bussani R, Amin MS, Vetrovec GW, Baldi A. Acute myocardial infarction and heart failure: role of apoptosis. Int J Biochem Cell Biol. 2006; 38:1834–1840.
Article36. Zucchi R, Ghelardoni S, Evangelista S. Biochemical basis of ischemic heart injury and of cardioprotective interventions. Curr Med Chem. 2007; 14:1619–1637.
Article37. Cohen M, Boiangiu C, Abidi M. Therapy for ST-segment elevation myocardial infarction patients who present late or are ineligible for reperfusion therapy. J Am Coll Cardiol. 2010; 55:1895–1906.
Article38. Ahmad R, Javed S, Bhandari U. Antiapoptotic potential of herbal drugs in cardiovascular disorders: an overview. Pharm Biol. 2010; 48:358–374.
Article39. Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004; 95:957–970.
Article40. Fischbach MA, Sonnenburg JL. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe. 2011; 10:336–347.
Article41. Aprikian O, Duclos V, Guyot S, Besson C, Manach C, Bernalier A, Morand C, Rémésy C, Demigné C. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J Nutr. 2003; 133:1860–1865.
Article42. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999; 69:30–42.
Article43. Wu H, Dwyer KM, Fan Z, Shircore A, Fan J, Dwyer JH. Dietary fiber and progression of atherosclerosis: the Los Angeles Atherosclerosis Study. Am J Clin Nutr. 2003; 78:1085–1091.
Article44. Veldman FJ, Nair CH, Vorster HH, Vermaak WJ, Jerling JC, Oosthuizen W, Venter CS. Dietary pectin influences fibrin network structure in hypercholesterolaemic subjects. Thromb Res. 1997; 86:183–196.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Physiologic Concentration of Estrogen on Ischemia/Reperfusion-induced Apoptosis in Rat Myocardium
- Imaging Myocardial Ischemia and Reperfusion Injury via Cy5.5-Annexin V
- Supplementation with psyllium seed husk reduces myocardial damage in a rat model of ischemia/reperfusion
- Naloxone Postconditioning Alleviates Rat Myocardial Ischemia Reperfusion Injury by Inhibiting JNK Activity
- Effects of Ivabradine on Left Ventricular Systolic Function and Cardiac Fibrosis in Rat Myocardial Ischemia-Reperfusion Model