Lab Med Online.
2016 Jan;6(1):19-24. 10.3343/lmo.2016.6.1.19.
Performance Evaluation of the ichroma SMART Analyzer in Measuring C-reactive Protein and Procalcitonin Levels
- Affiliations
-
- 1Department of Laboratory Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea. jhyooken@gmail.com
- 2Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Catholic Kwandong University International St. Mary's Hospital, Incheon, Korea.
- KMID: 2312287
- DOI: http://doi.org/10.3343/lmo.2016.6.1.19
Abstract
- BACKGROUND
For monitoring infection and inflammation episodes, biomarkers of host response, such as C-reactive protein (CRP) and procalcitonin (PCT), are now being recognized as useful tools in the diagnostic process. We aimed at evaluating the analytical performance of the recently developed semi-automated ichroma SMART system (Boditech Med Inc., Korea), which allows measurements of both CRP and PCT.
METHODS
We evaluated the analytical performance of the ichroma SMART system and the agreement between its results and the laboratory standards for CRP and PCT measurements. The precision and linearity as well as the method of measurement were compared to the DxC 800 (Beckman Coulter, USA) for CRP and to the VIDAS (bioMerieux SA, France) for PCT, according to corresponding CLSI guidelines. Additionally, we evaluated the carryover rates between specimens.
RESULTS
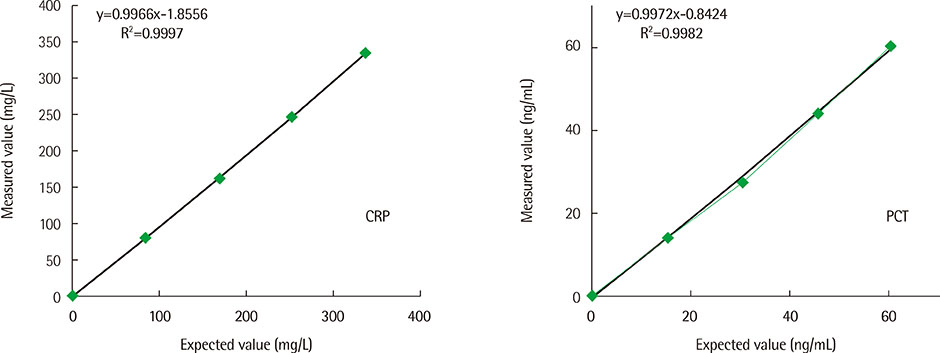
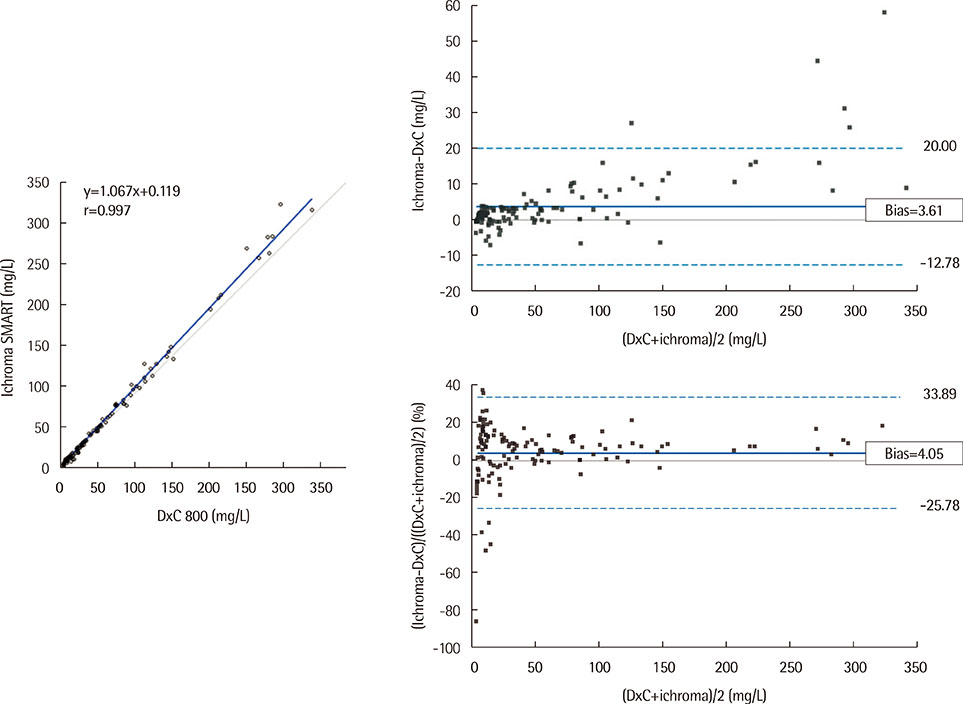
The total precision (% CV) of the ichroma SMART system in measuring low, middle, and high level controls (level 1, 2, 3) was 6.32%, 5.75%, and 3.56% for CRP, and 8.07%, 6.24%, and 6.53% for PCT. In the linearity test, R2 was 0.9997 and 0.9982 for CRP (0.1-336.8 mg/L) and PCT (0.05-60.91 ng/mL), respectively. Good correlation was observed between ichroma SMART and DxC 800 for CRP (r=0.997), and between ichroma SMART and VIDAS for PCT (r=0.992). Carry-over effect was 0.02% for CRP and 0.04% for PCT.
CONCLUSIONS
The ichroma SMART system showed an adequate performance and appeared to be a suitable clinical analyzer with a simple operating procedure for the measurement of CRP and PCT.
Keyword
Figure
Reference
-
1. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107:499–511.2. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993; 341:515–518.
Article3. Brouwer N, van Pelt J. Validation and evaluation of eight commercially available point of care CRP methods. Clin Chim Acta. 2015; 439:195–201.
Article4. Dipalo M, Buonocore R, Gnocchi C, Picanza A, Aloe R, Lippi G. Analytical evaluation of Diazyme procalcitonin (PCT) latex-enhanced immunoturbidimetric assay on Beckman Coulter AU5800. Clin Chem Lab Med. 2015; 53:593–597.
Article5. Clinical and Laboratory Standards Institute. Evaluation of Precision of Quantitative Measurement Procedures; Approved Guideline-Third Edition. EP05-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2014.6. Clinical and Laboratory Standards Institute. Evaluation of the Linearity of Quantitative Measurement Procedures: a Statistical Approach; Approved Guideline. EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.7. Clinical and Laboratory Standards Institute. Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Approved Guideline - Second Edition (Interim Revision). EP09-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2013.8. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999; 59:491–500.
Article9. Barassi A, Pallotti F, Melzi d'Eril G. Biological variation of procalcitonin in healthy individuals. Clin Chem. 2004; 50:1878.
Article10. Minnaard MC, van de Pol AC, Broekhuizen BD, Verheij TJ, Hopstaken RM, van Delft S, et al. Analytical performance, agreement and user-friendliness of five C-reactive protein point-of-care tests. Scand J Clin Lab Invest. 2013; 73:627–634.
Article11. Oh SW, Moon JD, Park SY, Jang HJ, Kim JH, Nahm KB, et al. Evaluation of fluorescence hs-CRP immunoassay for point-of-care testing. Clin Chim Acta. 2005; 356:172–177.
Article12. Kim KE, Han JY. Evaluation of the clinical performance of an automated procalcitonin assay for the quantitative detection of bloodstream infection. Korean J Lab Med. 2010; 30:153–159.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The prognostic value of procalcitonin, C-reactive protein and cholesterol in patients with an infection and multiple organ dysfunction
- Usefulness of Measuring Serum Procalcitonin Levels in Patients with Inflammatory Bowel Disease
- Performance Evaluation of Cartridge-Type Blood Gas Analyzer: i-Smart 300
- Serum Procalcitonin Level Reflects the Severity of Cellulitis
- Comparison of the diagnostic performance of initial serum procalcitonin, lactate, and C-reactive protein for predicting bacteremia in female patients with acute pyelonephritis