Lab Med Online.
2017 Jan;7(1):20-27. 10.3343/lmo.2017.7.1.20.
Performance Evaluation of Cartridge-Type Blood Gas Analyzer: i-Smart 300
- Affiliations
-
- 1Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea. ymyun@kuh.ac.kr
- KMID: 2363002
- DOI: http://doi.org/10.3343/lmo.2017.7.1.20
Abstract
- BACKGROUND
Blood gas analysis plays a crucial role in critical care settings, and immediate and precise analysis improves clinical outcomes through prompt treatment. We evaluated the performance of a cartridge-type blood gas analyzer, i-Smart 300 (i-SENS, Korea), according to the Clinical and Laboratory Standard Institute (CLSI) guidelines and compared it to a conventional blood gas analyzer.
METHODS
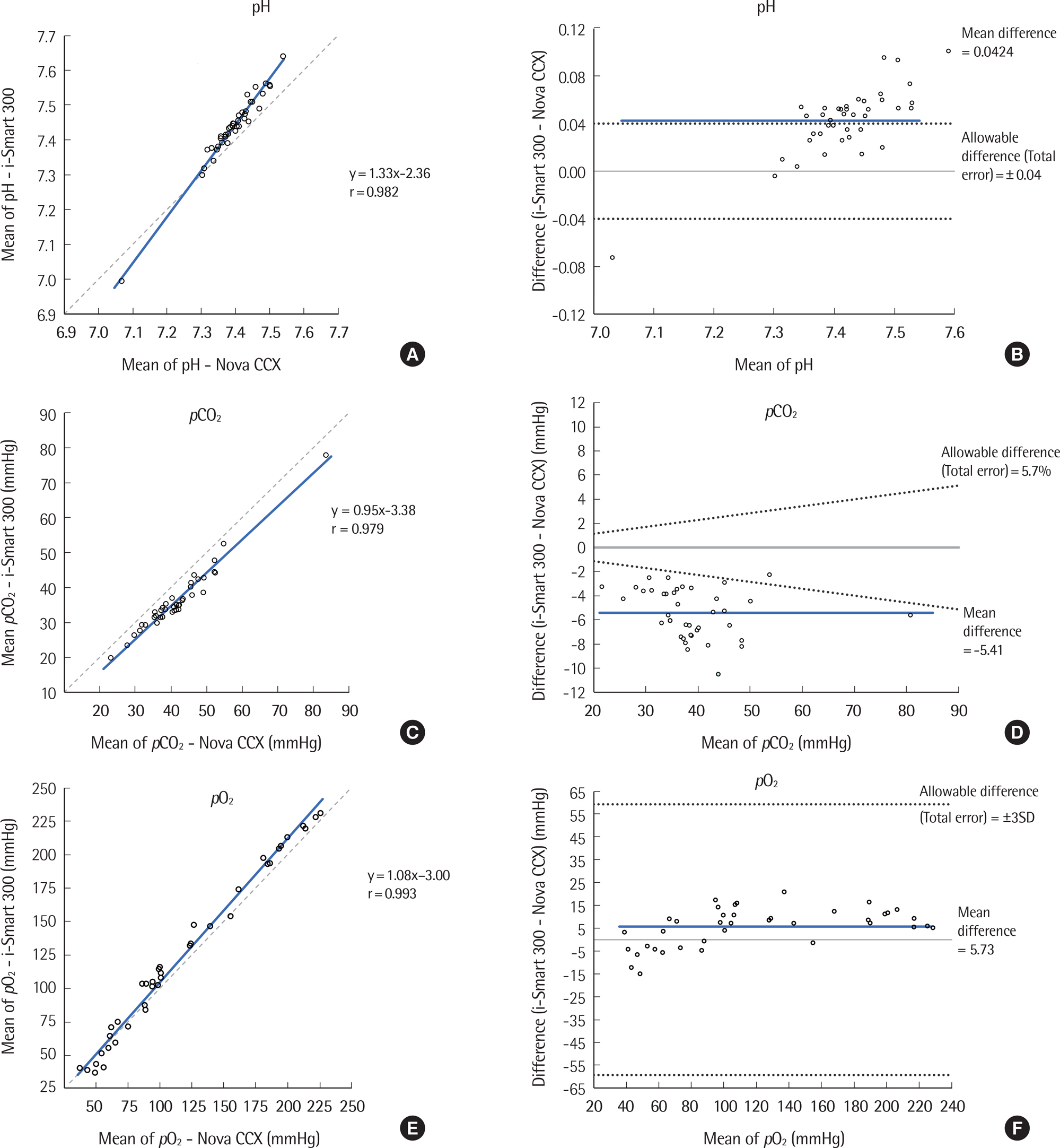
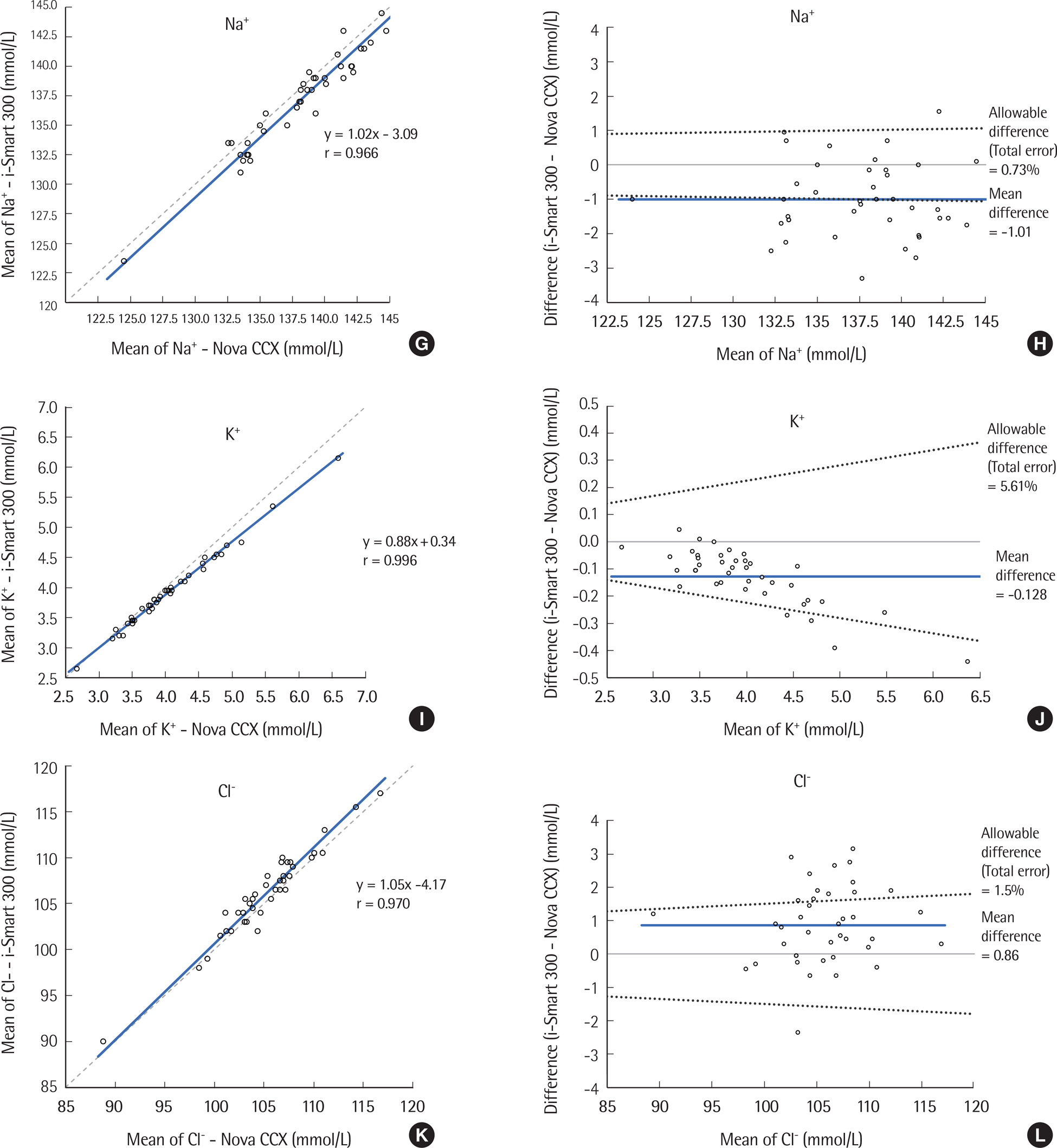
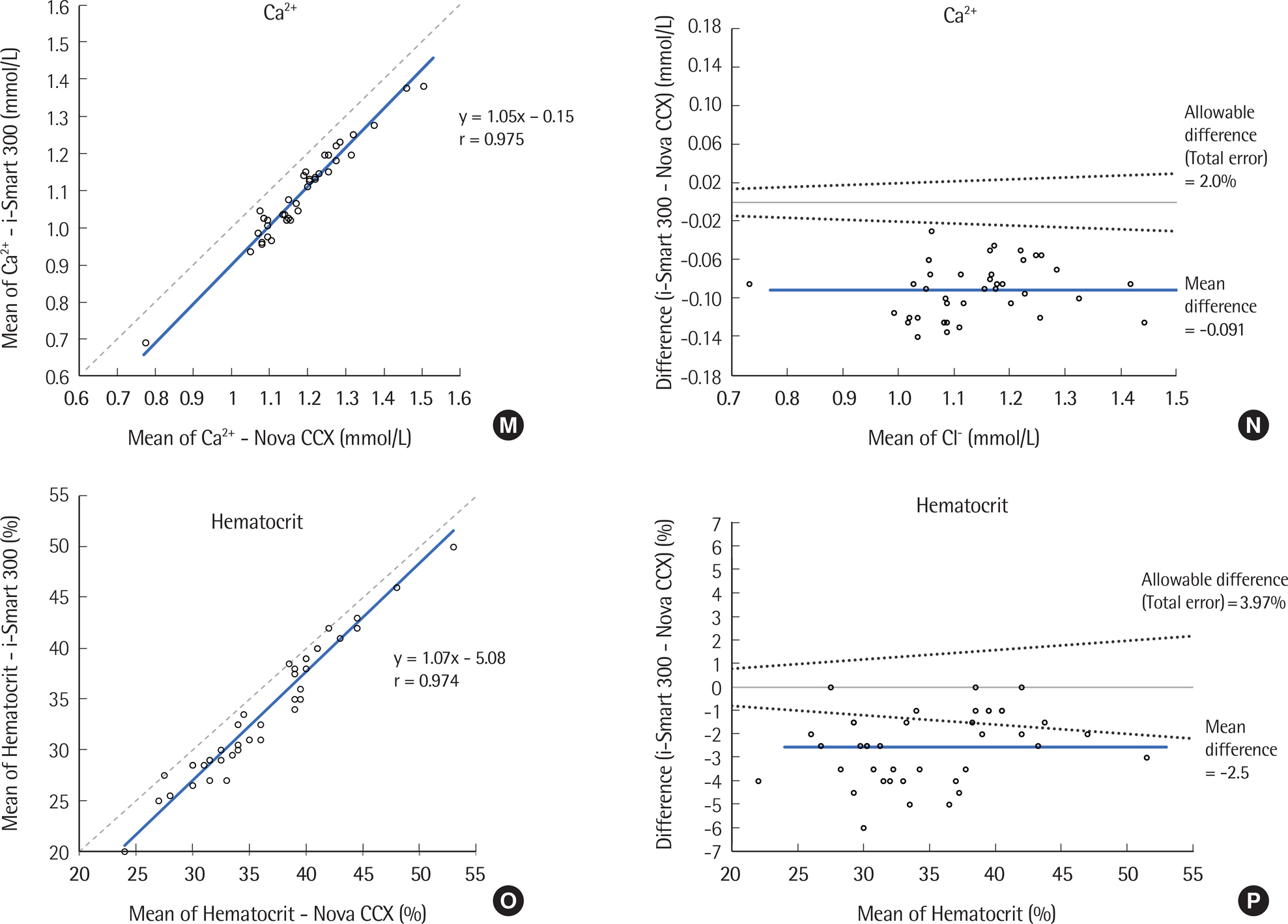
The precision was evaluated according to CLSI EP5-A3. The i-Smart 300 was compared to the Stat Profile Critical Care Xpress (STP CCX) (Nova CCX; Nova Biomedical, USA) according to CLSI EP9-A3 using the following eight parameters: pH, partial carbon dioxide pressure, partial oxygen pressure, sodium, potassium, chloride, ionized calcium, and hematocrit. Linearity was determined using five levels of control materials according to CLSI EP6-A.
RESULTS
Within-run precision and total precision, demonstrated as coefficients of variation, ranged from 0.02 to 2.50% and from 0.05 to 3.46%, respectively. Correlation analysis yielded a correlation coefficient from 0.966 to 0.996 between the i-Smart 300 and the conventional analyzer (Nova CCX). The i-Smart 300 showed excellent linearity at eight parameters with acceptable percent recovery.
CONCLUSIONS
The i-Smart 300, a portable cartridge-type blood gas analyzer, showed high precision and good correlation with a traditional bench-top blood gas analyzer. It could be useful in critical care settings.
MeSH Terms
Figure
Reference
-
1. Uyanik M, Sertoglu E, Kayadibi H, Tapan S, Serdar MA, Bilgi C, et al. Comparison of blood gas, electrolyte and metabolite results measured with two different blood gas analyzers and a core laboratory analyzer. Scand J Clin Lab Invest. 2015; 75:97–105.
Article2. De Koninck AS, De Decker K, Van Bocxlaer J, Meeus P, Van Hoovels L. Analytical performance evaluation of four cartridge-type blood gas analyzers. Clin Chem Lab Med. 2012; 50:1083–91.
Article3. Einstein MH, Smith KM, Davis TE, Schmeler KM, Ferris DG, Savage AH, et al. Clinical evaluation of the cartridge-based GeneXpert human papillomavirus assay in women referred for colposcopy. J Clin Microbiol. 2014; 52:2089–95.
Article4. Leino A, Kurvinen K. Interchangeability of blood gas, electrolyte and metabolite results measured with point-of-care, blood gas and core laboratory analyzers. Clin Chem Lab Med. 2011; 49:1187–91.
Article5. CLSI. Blood Gas and pH Analysis and Related Measurements; Approved Guideline—Second Edition. CLSI document C46-A2. Wayne, PA: Clinical and Laboratory Standards Institute. 2009.6. CLSI. Evaluation of Precision of Quantitative Measurement Procedures; Approved Guideline—Third Edition. CLSI document EP05-A3. Wayne, PA: Clinical and Laboratory Standards Institute. 2014.7. CLSI. Preliminary evaluation of quantitative clinical laboratory measurement procedures; Approved Guideline—Third Edition. CLSI document EP10-A3. Wayne, PA: Clinical and Laboratory Standards Institute. 2006.8. CLSI. Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline. CLSI document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute. 2003.9. CLSI. Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Approved Guideline—Third Edition. CLSI document EP09-A3. Wayne, PA: Clinical and Laboratory Standards Institute. 2013.10. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999; 59:491–500.11. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Desirable specifcation for total error, imprecision, and bias, derived from intra-and inter-individual biologic variation. http://www.westgard.com/biodatabase1.htm. (updated for 2014).12. Nichols JH, Christenson RH, Clarke W, Gronowski A, Hammett-Stabler CA, Jacobs E, et al. Executive summary. The National Academy of Clinical Biochemistry Laboratory Medicine Practice Guideline: evidence-based practice for point-of-care testing. Clin Chim Acta. 2007; 379:14–28.
Article13. Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci. 1989; 27:409–37.
Article14. Cotlove E, Harris EK, Williams GZ. Biological and analytic components of variation in longterm studies of serum constituents in normal subjects. 3. Physiological and medical implications. Clin Chem. 1970; 16:1028–32.15. Harris EK. Statistical principles underlying analytic goal-setting in clinical chemistry. Am J Clin Pathol. 1979; 72(2 Suppl):374–82.16. Fraser CG. General strategies to set quality specifcations for reliability performance characteristics. Scand J Clin Lab Invest. 1999; 59:487–90.17. Seok YM, Lee WH, Yoon SY, Won YC, Kwon OH. Evaluation of the i-Smart 30 Point-of-care analyzer for use in clinical laboratory settings. J Lab Med Qual Assur. 2011; 33:25–30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the i-Smart 30 Point-of-Care Analyzer for Use in Clinical Laboratory Settings
- Comparison of Conventional Chiron 348 pH/blood Gas/electrolytes Analyzer and i-STAT Portable Clinical Analyzer
- Performance Evaluation of the Stat Profile pHOx Ultra Blood Gas Analyzer
- Assessment of Blood Glucose Values Measured by Blood Gas Analyzer or Portable Glucometer
- Evaluation of the Blood Gas Analyzer GEM Premier 4000