Lab Med Online.
2011 Jan;1(1):10-18.
The Evaluation of Integrated Test as an Antenatal Screening Test for Down's Syndrome in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, CHA University, Seongnam, Korea. jiyoungh@cha.ac.kr
- 2Department of Obstetrics and Gyncology, CHA University, Seongnam, Korea.
Abstract
- BACKGROUND
Antenatal screening for Down's syndrome has been developed and improved over the past 20 yr. Recently, integrated test, which combines the first and second trimester markers has shown the highest detection rate (DR) and lowest false positive rate (FPR) among Down's syndrome screening tests currently in use. The purposes of this study were to evaluate the screening performance of integrated test and to compare the results with triple test studies in Korea.
METHODS
The study population consisted of Korean pregnant women who underwent triple or integrated test between April 2005 and December 2008. Triple test was performed using measurements of alpha-fetoprotein (AFP), unconjugated estriol (uE3), and human chorionic gonadotropin (hCG) in the second trimester. Integrated test was performed using nuchal translucency (NT) by ultrasonography and pregnancy-associated plasma protein A (PAPP-A) from maternal serum in the first trimester, and AFP, uE3, hCG, and inhibin-A in the second trimester. The screening performance of each test was evaluated by DR and FPR.
RESULTS
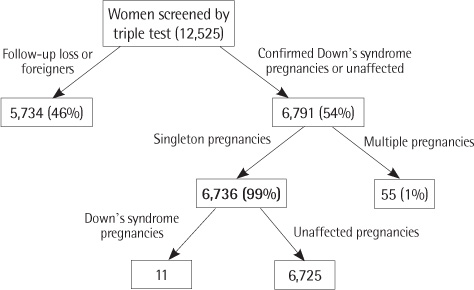
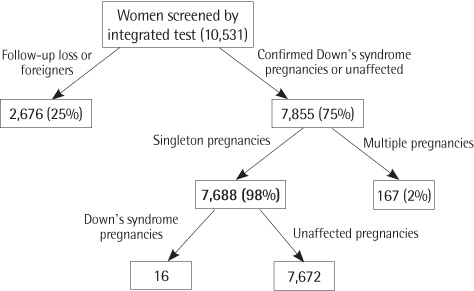
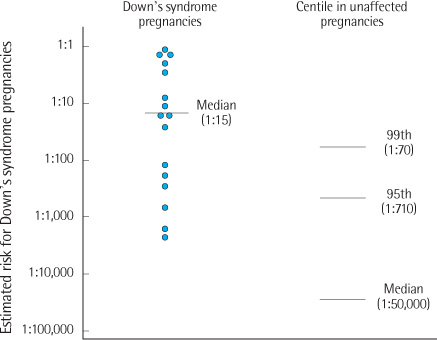
Twenty-seven Down's syndrome pregnancies were confirmed in women screened by triple (N=6,736) or integrated test (N=7,688). At 1:100, 1:270, and 1:300 of risk cutoff, triple test showed 45%, 73%, and 73% of DR and 4.7%, 11.2%, and 12.4% of FPR, respectively. At 1:100, 1:150, and 1:300 of risk cutoff, integrated test showed 63%, 69%, and 75% of DR and 1.5%, 1.9%, and 3.0% of FPR, respectively.
CONCLUSIONS
Integrated test showed higher DR and lower FPR, demonstrating better screening performance than triple test.
MeSH Terms
-
alpha-Fetoproteins
Chorionic Gonadotropin
Down Syndrome
Estriol
Female
Humans
Korea
Mass Screening
Nuchal Translucency Measurement
Plasma
Pregnancy
Pregnancy Trimester, First
Pregnancy Trimester, Second
Pregnant Women
Prenatal Diagnosis
Staphylococcal Protein A
Chorionic Gonadotropin
Estriol
Staphylococcal Protein A
alpha-Fetoproteins
Figure
Reference
-
1. Cuckle HS, Wald NJ, Lindenbaum RH. Maternal serum alpha-fetoprotein measurement: a screening test for Down syndrome. Lancet. 1984. 1:926–929.
Article2. Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Royston P, Chard T, et al. Maternal serum screening for Down's syndrome in early pregnancy. BMJ. 1988. 297:883–887.
Article3. Van Lith JM, Pratt JJ, Beekhuis JR, Mantingh A. Second-trimester maternal serum immunoreactive inhibin as a marker for fetal Down's syndrome. Prenat Diagn. 1992. 12:801–806.
Article4. Wald NJ, Densem JW, George L, Muttukrishna S, Knight PG. Prenatal screening for Down's syndrome using inhibin-A as a serum marker. Prenat Diagn. 1996. 16:143–153.
Article5. Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005. 353:2001–2011.
Article6. Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Downs syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003. 10:56–104.
Article7. Malone FD, D'Alton ME. First-trimester sonographic screening for Down syndrome. Obstet Gynecol. 2003. 102:1066–1079.
Article8. Nicolaides KH, Snijders RJ, Gosden CM, Berry C, Campbell S. Ultrasonographically detectable markers of fetal chromosomal abnormalities. Lancet. 1992. 340:704–707.
Article9. Brizot ML, Snijders RJ, Bersinger NA, Kuhn P, Nicolaides KH. Maternal serum pregnancy-associated plasma protein A and fetal nuchal translucency thickness for the prediction of fetal trisomies in early pregnancy. Obstet Gynecol. 1994. 84:918–922.10. Spencer K, Macri JN, Aitken DA, Connor JM. Free beta-hCG as first-trimester marker for fetal trisomy. Lancet. 1992. 339:1480.11. Wald NJ, Rish S, Hackshaw AK. Combining nuchal translucency and serum markers in prenatal screening for Down syndrome in twin pregnancies. Prenat Diagn. 2003. 23:588–592.
Article12. Wapner RJ. First trimester screening: the BUN study. Semin Perinatol. 2005. 29:236–239.
Article13. Bindra R, Heath V, Liao A, Spencer K, Nicolaides KH. One-stop clinic for assessment of risk for trisomy 21 at 11-14 weeks: a prospective study of 15,030 pregnancies. Ultrasound Obstet Gynecol. 2002. 20:219–225.
Article14. Kim S, Kim YH, Min WK. Prenatal serum marker screening in Korea: survey results. Korean J Lab Med. 2007. 27:28–33.
Article15. Hwang D, Park SH, Kim JY, Kim KC, Min EG. Prenatal screening using maternal serum AFP, uE3 and hCG in Korean pregnant women: about the correction of MoM value according to maternal weight. Korean J Obstet Gynecol. 1995. 38:378–388.16. Dallaire L. Integration of prenatal diagnosis of genetic diseases into medical practice. Can Med Assoc J. 1976. 115:713–714.17. Caron L, Tihy F, Dallaire L. Frequencies of chromosomal abnormalities at amniocentesis: over 20 years of cytogenetic analyses in one laboratory. Am J Med Genet. 1999. 82:149–154.
Article18. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. ACOG educational bulletin. Maternal serum screening. Number 228, September 1996 (replaces no. 154, April 1991). Int J Gynaecol Obstet. 1996. 55:299–308.19. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin No. 77: screening for fetal chromosomal abnormalities. Obstet Gynecol. 2007. 109:217–227.20. Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down's syndrome. J Med Screen. 1997. 4:181–246.
Article21. Pandya PP, Snijders RJ, Johnson SP, De Lourdes Brizot M, Nicolaides KH. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10 to 14 weeks of gestation. Br J Obstet Gynaecol. 1995. 102:957–962.
Article22. Snijders RJ, Johnson S, Sebire NJ, Noble PL, Nicolaides KH. First-trimester ultrasound screening for chromosomal defects. Ultrasound Obstet Gynecol. 1996. 7:216–226.
Article23. Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10-14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet. 1998. 352:343–346.
Article24. Taipale P, Hiilesmaa V, Salonen R, Ylöstalo P. Increased nuchal translucency as a marker for fetal chromosomal defects. N Engl J Med. 1997. 337:1654–1658.
Article25. Cuckle HS, Wald NJ, Thompson SG. Estimating a woman's risk of having a pregnancy associated with Down's syndrome using her age and serum alpha-fetoprotein level. Br J Obstet Gynaecol. 1987. 94:387–402.
Article26. Wald NJ, Hackshaw AK, George LM. Assay precision of serum alpha fetoprotein in antenatal screening for neural tube defects and Down's syndrome. J Med Screen. 2000. 7:74–77.
Article27. Cho HJ, Lee SY, Kim JW. Determination of Reference Level for Triple Marker in Korean Population. J Lab Med Qual Assur. 2004. 26:185–192.28. Spencer K, Cicero S, Atzei A, Otigbah C, Nicolaides KH. The influence of maternal insulin-dependent diabetes on fetal nuchal translucency thickness and first-trimester maternal serum biochemical markers of aneuploidy. Prenat Diagn. 2005. 25:927–929.
Article29. Webster RA. McPherson RA, Pincus MR, editors. Reproductive function and pregnancy. Henry's clinical diagnosis and management by laboratory methods. 2007. 21st ed. Philadelphia: Saunders Elsevier;373.30. O'Brien JE, Dvorin E, Drugan A, Johnson MP, Yaron Y, Evans MI. Race-ethnicity-specific variation in multiple-marker biochemical screening: alpha-fetoprotein, hCG, and estriol. Obstet Gynecol. 1997. 89:355–358.31. Crossley JA, Aitken DA, Waugh SM, Kelly T, Connor JM. Maternal smoking: age distribution, levels of alpha-fetoprotein and human chorionic gonadotropin, and effect on detection of Down syndrome pregnancies in second-trimester screening. Prenat Diagn. 2002. 22:247–255.
Article32. Dashe JS, Twickler DM, Santos-Ramos R, McIntire DD, Ramus RM. Alpha-fetoprotein detection of neural tube defects and the impact of standard ultrasound. Am J Obstet Gynecol. 2006. 195:1623–1628.
Article33. Kagan KO, Wright D, Maiz N, Pandeva I, Nicolaides KH. Screening for trisomy 18 by maternal age, fetal nuchal translucency, free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2008. 32:488–492.
Article34. Breathnach FM, Malone FD, Lambert-Messerlian G, Cuckle HS, Porter TF, Nyberg DA, et al. First- and second-trimester screening: detection of aneuploidies other than Down syndrome. Obstet Gynecol. 2007. 110:651–657.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The recent trend of prenatal screening
- A Research on the Actual Condition of Antenatal Screening Application in Obstetric Clinics in Korea and Suggestions for Preparation of Antenatal Screening Guideline
- Prenatal maternal serum screening for fetal aneuploidy
- The Carpal compression Test for Diagnosing Carpal Tunnel Syndrome
- Clinical analysis of triple marker screening test for fetal Down syndrome in midtrimester of pregnancy-Low sensitivity of triple marker screening test