Lab Anim Res.
2015 Dec;31(4):188-197. 10.5625/lar.2015.31.4.188.
Tissue transglutaminase-interleukin-6 axis facilitates peritoneal tumor spreading and metastasis of human ovarian cancer cells
- Affiliations
-
- 1PharmAbcine, Inc., #402 DaejeonBioventure Town, Jeonmin-dong, Yusung-gu, Daejeon, Korea.
- 2Laboratory of Immunology/Cancer Biology, Department of Biomedical Sciences, Seoul National University College of Medicine, 103 Daehakro, Jongno-gu, Seoul 110-799, Korea.
- 3Cancer Research Institute, Interdisciplinary Program of Tumor Biology, Seoul National University College of Medicine, 103 Daehakro, Jongno-gu, Seoul 110-799, Korea.
- 4Transplantation Research Institute, Seoul National University College of Medicine, 103 Daehakro, Jongno-gu, Seoul 110-799, Korea.
- 5Department of Surgery, Seoul National University College of Medicine, 103 Daehakro, Jongno-gu, Seoul 110-799, Korea.
- 6Department of Anatomy, Dankook University College of Medicine, 119 Dandaero, Dongnam-gu, Cheonan, Chungnam 31116, Korea. ybyoo36@hanmail.net
- KMID: 2312145
- DOI: http://doi.org/10.5625/lar.2015.31.4.188
Abstract
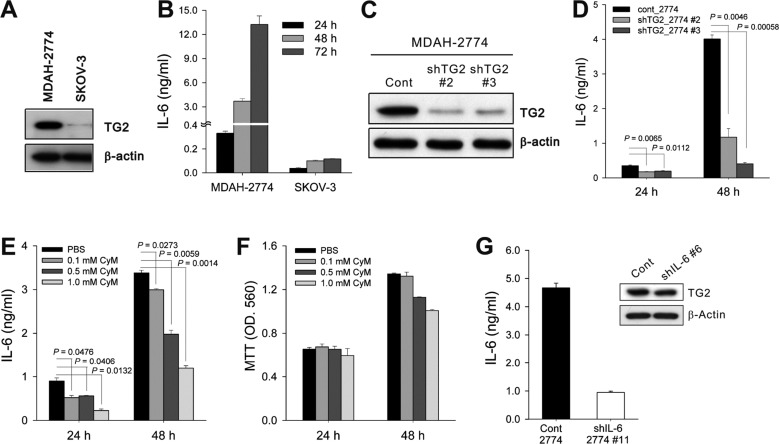
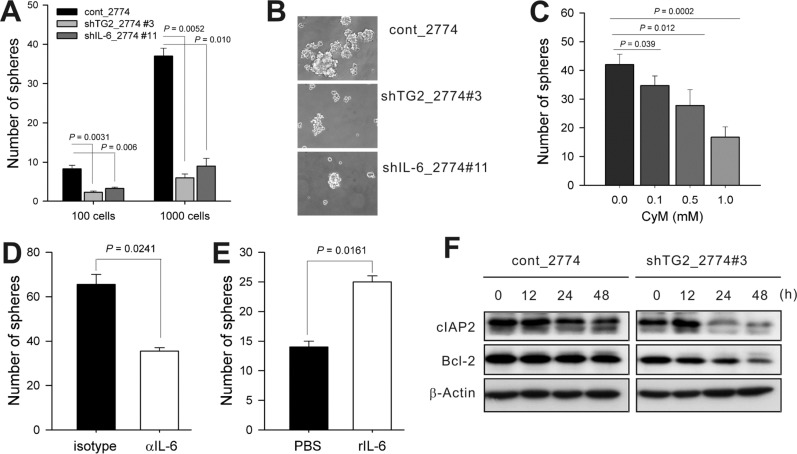
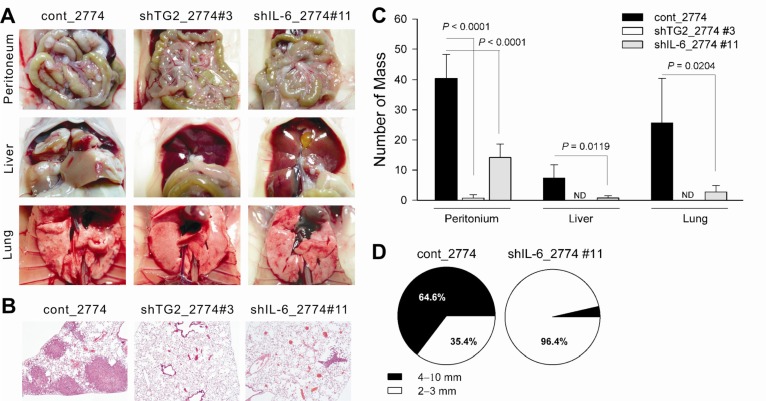
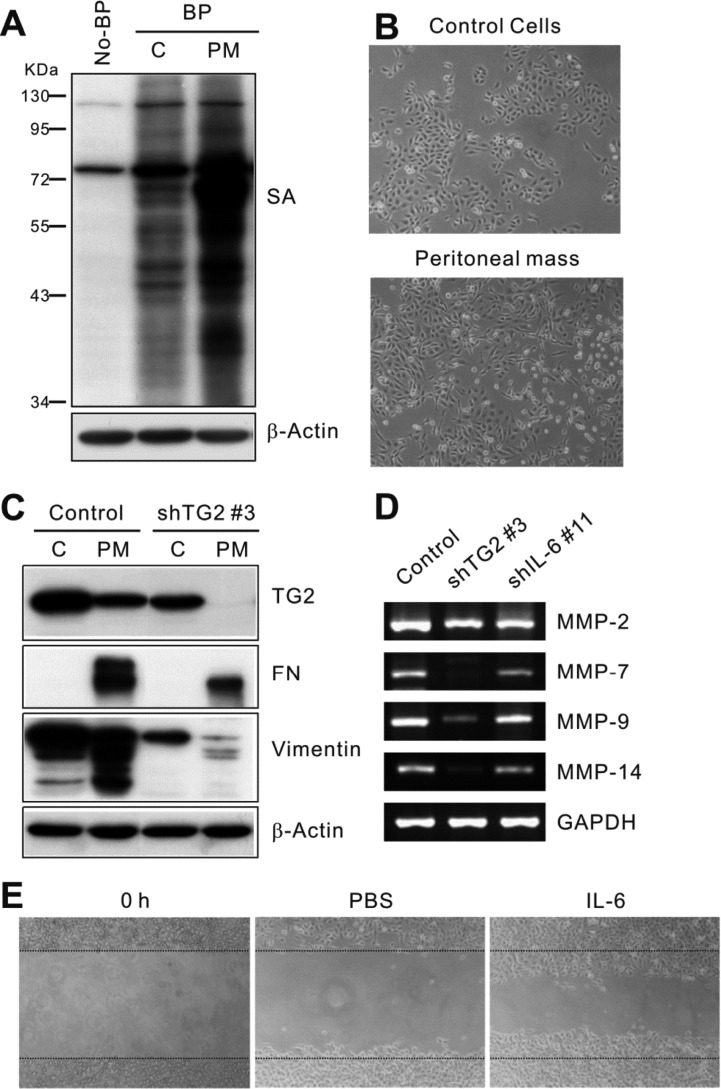
- Inflammation has recently been implicated in cancer formation and progression. As tissue transglutaminase (TG2) has been associated with both inflammatory signaling and tumor cell behavior, we propose that TG2 may be an important link inducing interleukin-6 (IL-6)-mediated cancer cell aggressiveness, including cancer stem cell-like characteristics and distant hematogenous metastasis. We evaluated the effect of differential TG2 and IL-6 expression on in vivo distant metastasis of human ovarian cancer cells. IL-6 production in human ovarian cancer cells was dependent on their TG2 expression levels. The size and efficiency of tumor sphere formation were correlated with TG2 expression levels and were dependent on TG2-mediated IL-6 secretion in human ovarian cancer cells. Primary tumor growth and propagation in the peritoneum and distant hematogenous metastasis into the liver and lung were also dependent on TG2 and downstream IL-6 expression levels in human ovarian cancer cells. In this report, we provide evidence that TG2 is an important link in IL-6-mediated tumor cell aggressiveness, and that TG2 and downstream IL-6 could be important mediators of distant hematogenous metastasis of human ovarian cancer cells. Intervention specific to TG2 and/or downstream IL-6 in ovarian cancer cells could provide a promising means to control tumor metastasis.
MeSH Terms
Figure
Reference
-
1. Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003; 4(2):140–156. PMID: 12563291.
Article2. Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002; 27(10):534–539. PMID: 12368090.
Article3. Mehta K. Biological and therapeutic significance of tissue transglutaminase in pancreatic cancer. Amino Acids. 2009; 36(4):709–716. PMID: 18594944.
Article4. Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res. 2004; 10(23):8068–8076. PMID: 15585642.
Article5. Verma A, Mehta K. Tissue transglutaminase-mediated chemoresistance in cancer cells. Drug Resist Updat. 2007; 10(4-5):144–151. PMID: 17662645.
Article6. Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, Mehta K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006; 66(21):10525–10533. PMID: 17079475.
Article7. Antonyak MA, Miller AM, Jansen JM, Boehm JE, Balkman CE, Wakshlag JJ, Page RL, Cerione RA. Augmentation of tissue transglutaminase expression and activation by epidermal growth factor inhibit doxorubicin-induced apoptosis in human breast cancer cells. J Biol Chem. 2004; 279(40):41461–41467. PMID: 15272014.
Article8. Ai L, Kim WJ, Demircan B, Dyer LM, Bray KJ, Skehan RR, Massoll NA, Brown KD. The transglutaminase 2 gene (TGM2), a potential molecular marker for chemotherapeutic drug sensitivity, is epigenetically silenced in breast cancer. Carcinogenesis. 2008; 29(3):510–518. PMID: 18174247.9. Chi DS, Sabbatini P. Advanced ovarian cancer. Curr Treat Options Oncol. 2000; 1(2):139–146. PMID: 12057051.
Article10. Hwang JY, Mangala LS, Fok JY, Lin YG, Merritt WM, Spannuth WA, Nick AM, Fiterman DJ, Vivas-Mejia PE, Deavers MT, Coleman RL, Lopez-Berestein G, Mehta K, Sood AK. Clinical and biological significance of tissue transglutaminase in ovarian carcinoma. Cancer Res. 2008; 68(14):5849–5858. PMID: 18632639.
Article11. Satpathy M, Cao L, Pincheira R, Emerson R, Bigsby R, Nakshatri H, Matei D. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007; 67(15):7194–7202. PMID: 17671187.
Article12. Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis. 2008; 29(10):1893–1900. PMID: 18667446.
Article13. Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene. 2007; 26(17):2459–2470. PMID: 17043648.14. Verma A, Guha S, Wang H, Fok JY, Koul D, Abbruzzese J, Mehta K. Tissue transglutaminase regulates focal adhesion kinase/AKT activation by modulating PTEN expression in pancreatic cancer cells. Clin Cancer Res. 2008; 14(7):1997–2005. PMID: 18381937.
Article15. Antonyak MA, Li B, Regan AD, Feng Q, Dusaban SS, Cerione RA. Tissue transglutaminase is an essential participant in the epidermal growth factor-stimulated signaling pathway leading to cancer cell migration and invasion. J Biol Chem. 2009; 284(27):17914–17925. PMID: 19403524.
Article16. Kim DS, Park SS, Nam BH, Kim IH, Kim SY. Reversal of drug resistance in breast cancer cells by transglutaminase 2 inhibition and nuclear factor-kappaB inactivation. Cancer Res. 2006; 66(22):10936–10943. PMID: 17108131.17. Mann AP, Verma A, Sethi G, Manavathi B, Wang H, Fok JY, Kunnumakkara AB, Kumar R, Aggarwal BB, Mehta K. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006; 66(17):8788–8795. PMID: 16951195.18. Jang GY, Jeon JH, Cho SY, Shin DM, Kim CW, Jeong EM, Bae HC, Kim TW, Lee SH, Choi Y, Lee DS, Park SC, Kim IG. Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells. Oncogene. 2010; 29(3):356–367. PMID: 19838207.19. Lee J, Kim YS, Choi DH, Bang MS, Han TR, Joh TH, Kim SY. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J Biol Chem. 2004; 279(51):53725–53735. PMID: 15471861.20. Knüpfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat. 2007; 102(2):129–135. PMID: 16927176.
Article21. Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003; 88(11):1721–1726. PMID: 12771987.
Article22. Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009; 15(2):103–113. PMID: 19185845.23. Huber MA, Azoitei N, Baumann B, Grünert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004; 114(4):569–581. PMID: 15314694.24. Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009; 28(33):2940–2947. PMID: 19581928.
Article25. Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafè M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007; 117(17):3988–4002. PMID: 18060036.
Article26. Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003; 3(12):895–902. PMID: 14737120.
Article27. Oh K, Park HB, Byoun OJ, Shin DM, Jeong EM, Kim YW, Kim YS, Melino G, Kim IG, Lee DS. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J Exp Med. 2011; 208(8):1707–1719. PMID: 21746810.
Article28. Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008; 9(8):628–638. PMID: 18628784.
Article29. Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2009; 36(4):659–670. PMID: 18982407.
Article30. Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008; 133(4):704–715. PMID: 18485877.
Article31. Sodek KL, Ringuette MJ, Brown TJ. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int J Cancer. 2009; 124(9):2060–2070. PMID: 19132753.
Article32. Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002; 100(9):3175–3182. PMID: 12384415.33. Verderio EA, Telci D, Okoye A, Melino G, Griffin M. A novel RGD-independent cel adhesion pathway mediated by fibronectin-bound tissue transglutaminase rescues cells from anoikis. J Biol Chem. 2003; 278(43):42604–42614. PMID: 12732629.34. Satpathy M, Shao M, Emerson R, Donner DB, Matei D. Tissue transglutaminase regulates matrix metalloproteinase-2 in ovarian cancer by modulating cAMP-response element-binding protein activity. J Biol Chem. 2009; 284(23):15390–15399. PMID: 19324884.
Article35. Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008; 76(11):1352–1364. PMID: 18708031.
Article36. Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massagué J. Tumor self-seeding by circulating cancer cells. Cell. 2009; 139(7):1315–1326. PMID: 20064377.
Article37. Daniel D, Wilson NS. Tumor necrosis factor: renaissance as a cancer therapeutic? Curr Cancer Drug Targets. 2008; 8(2):124–131. PMID: 18336195.
Article38. Takahashi E, Nagano O, Ishimoto T, Yae T, Suzuki Y, Shinoda T, Nakamura S, Niwa S, Ikeda S, Koga H, Tanihara H, Saya H. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J Biol Chem. 2010; 285(6):4060–4073. PMID: 19965872.39. Chung YJ, Park BB, Kang YJ, Kim TM, Eaves CJ, Oh IH. Unique effects of Stat3 on the early phase of hematopoietic stem cell regeneration. Blood. 2006; 108(4):1208–1215. PMID: 16614239.
Article40. Müller S, Chakrapani BP, Schwegler H, Hofmann HD, Kirsch M. Neurogenesis in the dentate gyrus depends on ciliary neurotrophic factor and signal transducer and activator of transcription 3 signaling. Stem Cells. 2009; 27(2):431–441. PMID: 19023034.
Article41. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009; 139(5):871–890. PMID: 19945376.
Article42. Shao M, Cao L, Shen C, Satpathy M, Chelladurai B, Bigsby RM, Nakshatri H, Matei D. Epithelial-to-mesenchymal transition and ovarian tumor progression induced by tissue transglutaminase. Cancer Res. 2009; 69(24):9192–9201. PMID: 19951993.
Article43. Kumar A, Xu J, Brady S, Gao H, Yu D, Reuben J, Mehta K. Tissue transglutaminase promotes drug resistance and invasion by inducing mesenchymal transition in mammary epithelial cells. PLoS One. 2010; 5(10):e13390. PMID: 20967228.
Article44. Yuan L, Siegel M, Choi K, Khosla C, Miller CR, Jackson EN, Piwnica-Worms D, Rich KM. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene. 2007; 26(18):2563–2573. PMID: 17099729.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential diagnosis of ovarian cancer and benign ovarian diseases using cytokine levels in the peritoneal fluids
- Peritoneal Fluid levels of various Cytokines in Patients with Ovarian Lesions
- A Recurrence of Ovarian Carcinoma Presenting as Only Axillary Lymphatic Metastasis: A Case Report
- Diagnostic Efficiency of Peritoneal Fluid and Serum Lactate Dehydrogenase(LDH) in Ovarian Cancer Patients
- Regulation of Tumor Immune Surveillance and Tumor Immune Subversion by TGF-beta