Lab Anim Res.
2012 Mar;28(1):61-66. 10.5625/lar.2012.28.1.61.
Molecular identification of Mycoplasma cynos from laboratory beagle dogs with respiratory disease
- Affiliations
-
- 1Center for Animal Resources Development, Wonkwang University, Iksan, Korea. kimoj@wku.ac.kr
- 2Institute of Animal Experiment & Efficacy Evaluation, Wonkwang University, Iksan, Korea.
- 3Digestive Disease Research Institute, Wonkwang University, Iksan, Korea.
- KMID: 2312094
- DOI: http://doi.org/10.5625/lar.2012.28.1.61
Abstract
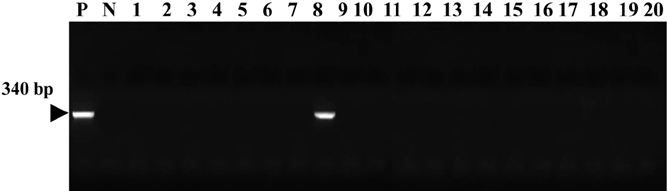
- In this study, we examined a colony of 20 beagle dogs in a laboratory animal facility. Mycoplasma was detected by consensus PCR assay in 1 dog with respiratory and constitutional symptoms. None of the other dogs were affected. The dog was euthanized and necropsied. In postmortem examinations, gray or plum-colored gross lesions were found on the lung, most commonly in the apical and cardiac lobes. Some lesions showed clear demarcation and consolidation. Microscopic examination showed peribronchiolar lymphoid hyperplasia and interstitial thickening, lesions pathognomonic for mycoplasma pneumonia. To identify canine Mycoplasma species, we used species-specific PCR reactions for M. arginini, M. canis, M. cynos, M. edwardii, M. felis, M. gateae, M. maculosum, M. molare, M. opalescens, M. spumans, Mycoplasma sp. HRC 689, and M. collis. As the result, we identified Mycoplasma cynos by amplification of DNA extracted from lung tissue of the laboratory beagle dog with respiratory disease.
Keyword
MeSH Terms
Figure
Reference
-
1. McAuliffe L, Ellis RJ, Ayling RD, Nicholas RA. Differentiation of Mycoplasma species by 16S ribosomal DNA PCR and denaturing gradient gel electrophoresis fingerprinting. J Clin Microbiol. 2003. 41(10):4844–4847.2. Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory Animal Medicine. 2002. 2nd ed. Amsterdam: Academic Press;111–165.3. Lindsey JR, Baker HJ, Overcash RG, Cassell GH, Hunt CE. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971. 64(3):675–708.4. Davis JK, Thorp RB, Maddox PA, Brown MB, Cassell GH. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982. 36(2):720–729.5. Rosendal S. Canine mycoplasmas: their ecologic niche and role in disease. J Am Vet Med Assoc. 1982. 180(10):1212–1214.6. Randolph JF, Moise NS, Scarlett JM, Shin SJ, Blue JT, Bookbinder PR. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and prevalence of mycoplasmal recovery from pharyngeal swab specimens in dogs with or without pulmonary disease. Am J Vet Res. 1993. 54(3):387–391.7. Armstrong D, Morton V, Friedman MH, Steger L, Tully JG. Canine pneumonia associated with mycoplasma infection. Am J Vet Res. 1972. 33(7):1471–1478.8. Rosendal S. Canine mycoplasmas: pathogenicity of mycoplasmas associated with distemper pneumonia. J Infect Dis. 1978. 138(2):203–210.9. Rosendal S. Mycoplasmas as a possible cause of enzootic pneumonia in dogs. Acta Vet Scand. 1972. 13(1):137–139.10. Rosendal S, Vinther O. Experimental mycoplasmal pneumonia in dogs: electron microscopy of infected tissue. Acta Pathol Microbiol Scand B. 1977. 85B(6):462–465.11. Chandler JC, Lappin MR. Mycoplasmal respiratory infections in small animals: 17 cases (1988-1999). J Am Anim Hosp Assoc. 2002. 38(2):111–119.12. Harasawa R, Koshimizu K, Takeda O, Uemori T, Asada K, Kato I. Detection of Mycoplasma hyopneumoniae DNA by the polymerase chain reaction. Mol Cell Probes. 1991. 5(2):103–109.13. Cho SJ, Lee HA, Hong S, Kim O. Uterine adenocarcinoma with feline leukemia virus infection. Lab Anim Res. 2011. 27(4):347–351.14. Hong S, Lee HA, Park SH, Kim O. Sensitive and Specific Detection of Mycoplasma species by Consensus Polymerase Chain Reaction and Dot Blot Hybridization. Lab Anim Res. 2011. 27(2):141–145.15. Chalker VJ, Brownlie J. Taxonomy of the canine Mollicutes by 16S rRNA gene and 16S/23S rRNA intergenic spacer region sequence comparison. Int J Syst Evol Microbiol. 2004. 54(Pt 2):537–542.16. Chalker VJ, Owen WM, Paterson C, Barker E, Brooks H, Rycroft AN, Brownlie J. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004. 150(Pt 10):3491–3497.17. van Kuppeveld FJ, van der Logt JT, Angulo AF, van Zoest MJ, Quint WG, Niesters HG, Galama JM, Melchers WJ. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl Environ Microbiol. 1992. 58(8):2606–2615.18. Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. 1999. 37(3):620–627.19. Cassell GH, Lindsey JR, Davis JK. Respiratory and genital mycoplasmosis of laboratory rodents: implications for biomedical research. Isr J Med Sci. 1981. 17(7):548–554.20. Aguila HN, Lai WC, Lu YS, Pakes SP. Experimental Mycoplasma pulmonis infection of rats suppresses humoral but not cellular immune response. Lab Anim Sci. 1988. 38(2):138–142.21. Faulkner CB, Simecka JW, Davidson MK, Davis JK, Schoeb TR, Lindsey JR, Everson MP. Gene expression and production of tumor necrosis factor alpha, interleukin 1, interleukin 6, and gamma interferon in C3H/HeN and C57BL/6N mice in acute Mycoplasma pulmonis disease. Infect Immun. 1995. 63(10):4084–2935.22. Mikola I, Balogh G, Nagy A, Mátyás M, Glávits R, Stipkovits L. Mycoplasma pneumoniae epidemic as zoonosis. Orv Hetil. 1997. 138(46):2933–2935.23. Rosengarten R, Citti C, Much P, Spergser J, Droesse M, Hewicker-Trautwein M. The changing image of mycoplasmas: from innocent bystanders to emerging and reemerging pathogens in human and animal diseases. Contrib Microbiol. 2001. 8:166–185.24. Greig AS. The Significance Of A Pleuropneumonia-Like Organism In Kennel Cough. Can J Comp Med Vet Sci. 1954. 18(8):275–279.25. Johansson K-E, Pettersson B. Razin S, Herrmann R, editors. Taxonomy of Mollicutes. Molecular Biology and Pathogenicity of Mycoplasmas. 2002. New York: Kluwer;1–27.26. Hill AC. Mycoplasma collis, a new species isolated from rats and mice. Int J Syst Bacteriol. 1983. 33(4):847–851.27. Kirchner BK, Port CD, Magoc TJ, Sidor MA, Ruben Z. Spontaneous bronchopneumonia in laboratory dogs infected with untyped Mycoplasma spp. Lab Anim Sci. 1990. 40(6):625–628.28. Kaklamanis E, Pavlatos M. The immunosuppressive effect of mycoplasma infection. I. Effect on the humoral and cellular response. Immunology. 1972. 22(4):695–702.29. Chvala S, Benetka V, Mostl K, Zeugswetter F, Spergser J, Weissenbock H. Simultaneous canine distemper virus, canine adenovirus type 2, and Mycoplasma cynos infection in a dog with pneumonia. Vet Pathol. 2007. 44(4):508–512.30. Zeugswetter F, Weissenbock H, Shibly S, Hassan J, Spergser J. Lethal bronchopneumonia caused by Mycoplasma cynos in a litter of golden retriever puppies. Vet Rec. 2007. 161(18):626–627.31. Rosendal S. Canine mycoplasmas. I. Cultivation from conjunctivae, respiratory- and genital tracts. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973. 81(4):441–445.32. Nagatomo H, Takegahara Y, Sonoda T, Yamaguchi A, Uemura R, Hagiwara S, Sueyoshi M. Comparative studies of the persistence of animal mycoplasmas under different environmental conditions. Vet Microbiol. 2001. 82(3):223–232.33. McAuliffe L, Ellis RJ, Miles K, Ayling RD, Nicholas RA. Biofilm formation by mycoplasma species and its role in environmental persistence and survival. Microbiology. 2006. 152(Pt 4):913–922.34. Chalker VJ, Toomey C, Opperman S, Brooks HW, Ibuoye MA, Brownlie J, Rycroft AN. Respiratory disease in kennelled dogs: serological responses to Bordetella bronchiseptica lipopolysaccharide do not correlate with bacterial isolation or clinical respiratory symptoms. Clin Diagn Lab Immunol. 2003. 10(3):352–356.