Lab Anim Res.
2012 Mar;28(1):31-38. 10.5625/lar.2012.28.1.31.
Modulation of lipid metabolism by mixtures of protamine and chitooligosaccharide through pancreatic lipase inhibitory activity in a rat model
- Affiliations
-
- 1Laboratory of Veterinary Biochemistry and Immunology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. kchoi@cbu.ac.kr
- 2LG Household & HealthCare Research Institute, Deajeon, Korea.
- KMID: 2312090
- DOI: http://doi.org/10.5625/lar.2012.28.1.31
Abstract
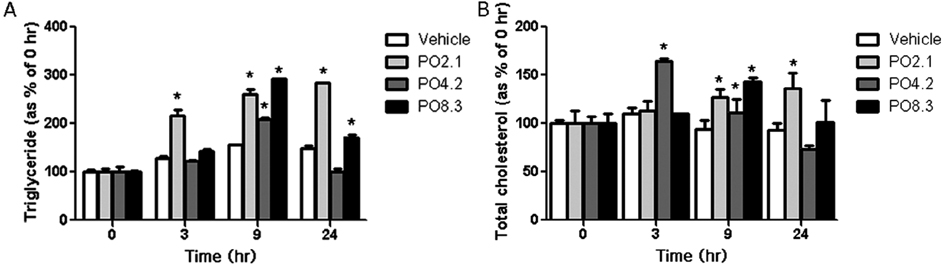
- Overweight and obesity are usually related with high fat and calorie intake, and seriously causative of lifestyle-related diseases such as cardiovascular disorders, arteriosclerosis, and colon cancer. In this study, we propose a novel dietary therapy against overweight and obesity using mixtures of protamine and chitooligosaccharide (COS), which are known to interrupt the lipid metabolism in the body. Protamine is a dietary protein originated from salmon reproductive organ, and COS is an oligosaccharide made from chitin or chitosan by chemical or enzymatic hydrolysis. In the enzyme activity analysis in vitro, protamine and COS strongly suppressed the activity of pancreatic lipase, which is the primary enzyme for the digestion and absorption of lipids in the intestine. In in vivo animal test, the mixtures of protamine and COS significantly reduced the serum levels of triglyceride (TG), total cholesterol (T-CHO), and low density lipoprotein-cholesterol (LDLC) and inhibited the accumulation of lipids in liver tissue of Sprague Dawley (SD) rats fed high fat diets. On the other hand, they increased fecal TG and T-CHO contents. From these alterations in lipid metabolism, we verified that protamine and COS mixtures could effectively interrupt the digestion and absorption of dietary lipids in the body by inhibiting pancreatic lipase activity. In addition, protamine and COS mixtures increased the serum level of high density lipoprotein-cholesterol (HDLC), responsible for removing cholesterol from cells and protecting atherosclerosis, and therefore decreased the potential risks of cardiovascular diseases by lowering values of the atherogenic index (AI) and cardiac risk factor (CRF). Taken together, we suggest protamine and COS mixtures as a prominent dietary therapy for the prevention of overweight, obesity, and further cardiovascular diseases related with hyperlipidemia.
MeSH Terms
-
Absorption
Animals
Arteriosclerosis
Atherosclerosis
Cardiovascular Diseases
Chitin
Chitosan
Cholesterol
Colonic Neoplasms
Diet, High-Fat
Dietary Proteins
Digestion
Hand
Hydrolysis
Hyperlipidemias
Intestines
Lipase
Lipid Metabolism
Liver
Obesity
Overweight
Rats
Risk Factors
Salmon
Chitin
Chitosan
Cholesterol
Dietary Proteins
Lipase
Figure
Reference
-
1. Pokorski R. Effect of increasing body weight on morbidity and mortality in South Korea. J Insur Med. 2011. 42(2-4):78–84.2. Bae JM, Yang YJ, Li ZM, Ahn YO. Low cholesterol is associated with mortality from cardiovascular diseases: a dynamic cohort study in Korean adults. J Korean Med Sci. 2012. 27(1):58–63.3. Hosomi R, Fukunaga K, Arai H, Kanda S, Nishiyama T, Yoshida M. Effect of dietary protamine on lipid metabolism in rats. Nutr Res Pract. 2010. 4(6):462–469.4. Aspedon A, Groisman EA. The antibacterial action of protamine: evidence for disruption of cytoplasmic membrane energization in Salmonella typhimurium. Microbiology. 1996. 142(Pt 12):3389–3397.5. Duarte-Vázquez MA, García-Padilla S, Olvera-Ochoa L, González-Romero KE, Acosta-Iñiguez J, De la Cruz-Cordero R, Rosado JL. Effect of protamine in obesity induced by high-fat diets in rats. Int J Obes (Lond). 2009. 33(6):687–692.6. Chae SY, Jang MK, Nah JW. Influence of molecular weight on oral absorption of water soluble chitosans. J Control Release. 2005. 102(2):383–394.7. Zhou TX, Chen YJ, Yoo JS, Huang Y, Lee JH, Jang HD, Shin SO, Kim HJ, Cho JH, Kim IH. Effects of chitooligosaccharide supplementation on performance, blood characteristics, relative organ weight, and meat quality in broiler chickens. Poult Sci. 2009. 88(3):593–600.8. Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH, Kim CY. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int J Antimicrob Agents. 2001. 18(6):553–557.9. Shon YH, Nam KS. Inhibition of polyamine biosynthesis in Acanthamoeba castellanii and 12-O-tetradecanoylphorbol-13-acetate-induced ornithine decarboxylase activity by chitosanoligosaccharide. Biotechnol Lett. 2003. 25(9):701–704.10. Cho SY, Lee JH, Song MJ, Park PJ, Shin ES, Sohn JH, Seo DB, Lim KM, Kim WG, Lee SJ. Effects of chitooligosaccharide lactate salt on sleep deprivation-induced fatigue in mice. Biol Pharm Bull. 2010. 33(7):1128–1132.11. Hayashi K, Ito M. Antidiabetic action of low molecular weight chitosan in genetically obese diabetic KK-Ay mice. Biol Pharm Bull. 2002. 25(2):188–192.12. Tang ZR, Yin YL, Nyachoti CM, Huang RL, Li TJ, Yang C, Yang XJ, Gong J, Peng J, Qi DS, Xing JJ, Sun ZH, Fan MZ. Effect of dietary supplementation of chitosan and galacto-mannan-oligosaccharide on serum parameters and the insulin-like growth factor-I mRNA expression in early-weaned piglets. Domest Anim Endocrinol. 2005. 28(4):430–441.13. Choi CR, Kim EK, Kim YS, Je JY, An SH, Lee JD, Wang JH, Ki SS, Jeon BT, Moon SH, Park PJ. Chitooligosaccharides decreases plasma lipid levels in healthy men. Int J Food Sci Nutr. 2012. 63(1):103–106.14. Nakai M, Fukui Y, Asami S, Toyoda-Ono Y, Iwashita T, Shibata H, Mitsunaga T, Hashimoto F, Kiso Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J Agric Food Chem. 2005. 53(11):4593–4598.15. Zhang J, Kang MJ, Kim MJ, Kim ME, Song JH, Lee YM, Kim JI. Pancreatic lipase inhibitory activity of taraxacum officinale in vitro and in vivo. Nutr Res Pract. 2008. 2(4):200–203.16. Tsujita T, Matsuura Y, Okuda H. Studies on the inhibition of pancreatic and carboxylester lipases by protamine. J Lipid Res. 1996. 37(7):1481–1487.17. Gors S, Kucia M, Langhammer M, Junghans P, Metges CC. Technical note: Milk composition in mice--methodological aspects and effects of mouse strain and lactation day. J Dairy Sci. 2009. 92(2):632–637.18. Hwang KA, Park SH, Yi BR, Choi KC. Gene alterations of ovarian cancer cells expressing estrogen receptors by estrogen and bisphenol a using microarray analysis. Lab Anim Res. 2011. 27(2):99–107.19. Yi BR, Kang NH, Hwang KA, Kim SU, Jeung EB, Choi KC. Antitumor therapeutic effects of cytosine deaminase and interferon-β against endometrial cancer cells using genetically engineered stem cells in vitro. Anticancer Res. 2011. 31(9):2853–2861.20. Hosomi R, Fukunaga K, Arai H, Nishiyama T, Yoshida M. Effects of dietary fish protein on serum and liver lipid concentrations in rats and the expression of hepatic genes involved in lipid metabolism. J Agric Food Chem. 2009. 57(19):9256–9262.21. Moriyama T, Kishimoto K, Nagai K, Urade R, Ogawa T, Utsumi S, Maruyama N, Maebuchi M. Soybean beta-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci Biotechnol Biochem. 2004. 68(2):352–359.22. Lowe ME. Molecular mechanisms of rat and human pancreatic triglyceride lipases. J Nutr. 1997. 127(4):549–557.23. Lowe ME. The triglyceride lipases of the pancreas. J Lipid Res. 2002. 43(12):2007–2016.24. Artenie R, Ungureanu D, Artenie A, Botnariu G, Anisie E. [HDL-cholesterol--active or passive participant in the pathogenesis of atherosclerosis]. Rev Med Chir Soc Med Nat Iasi. 2003. 107(2):282–287.25. Sancho-Rodriguez N, Aviles-Plaza FV, Granero-Fernandez E, Hernandez-Martinez AM, Albaladejo-Oton MD, Martinez-Mernandez P, Parra-Pallares S. Observational study of lipid profile and LDL particle size in patients with metabolic syndrome. Lipids Health Dis. 2011. 10:162.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro α-Amylase, α-Glucosidase, Pancreatic Lipase, Xanthine Oxidase Inhibiting Activity of Agaricus bisporus Extracts
- Extraction and Characteristics of Anti-obesity Lipase Inhibitor from Phellinus linteus
- Pancreatic lipase inhibitory activity of taraxacum officinale in vitro and in vivo
- Effect of dietary protamine on lipid metabolism in rats
- Evaluation of the Clinical Usefulness for Pancreatic Amylase in Acute Pancreatitis