Kosin Med J.
2012 Dec;27(2):79-89. 10.7180/kmj.2012.27.2.79.
Mini-review: Eosinophils, a Useful Diagnostic Clue in Surgical Neuropathology
- Affiliations
-
- 1Department of Pathology, Wonju College of Medicine, Yonsei University, Wonju, Korea.
- 2Department of Pathology, College of Medicine, Yonsei University, Seoul, Korea. paxco@yuhs.ac
- KMID: 2308515
- DOI: http://doi.org/10.7180/kmj.2012.27.2.79
Abstract
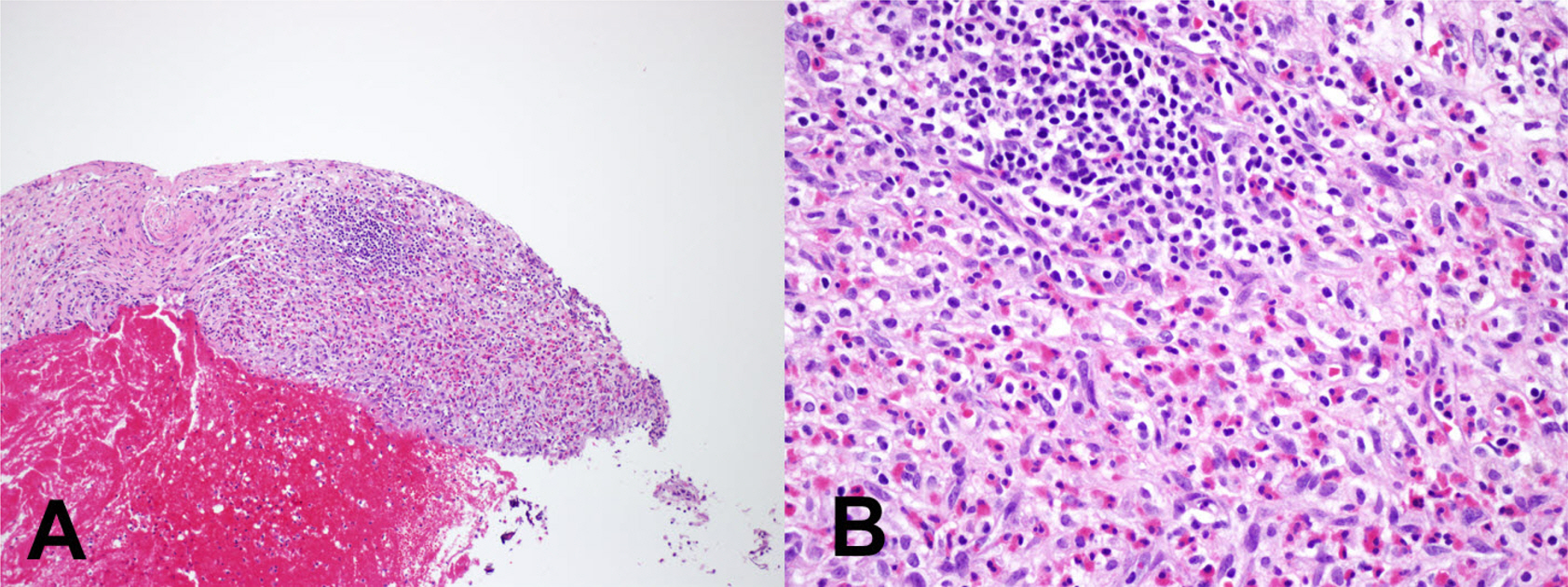
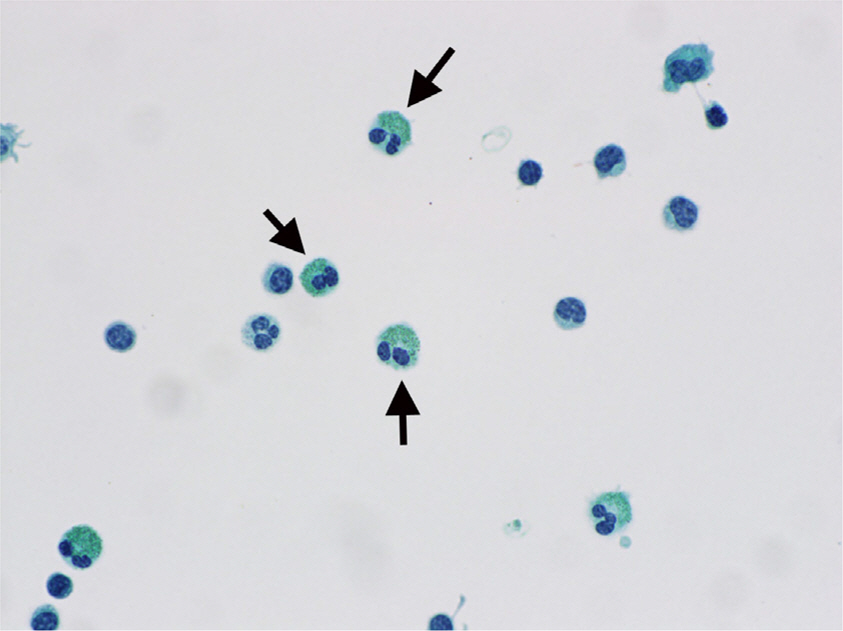
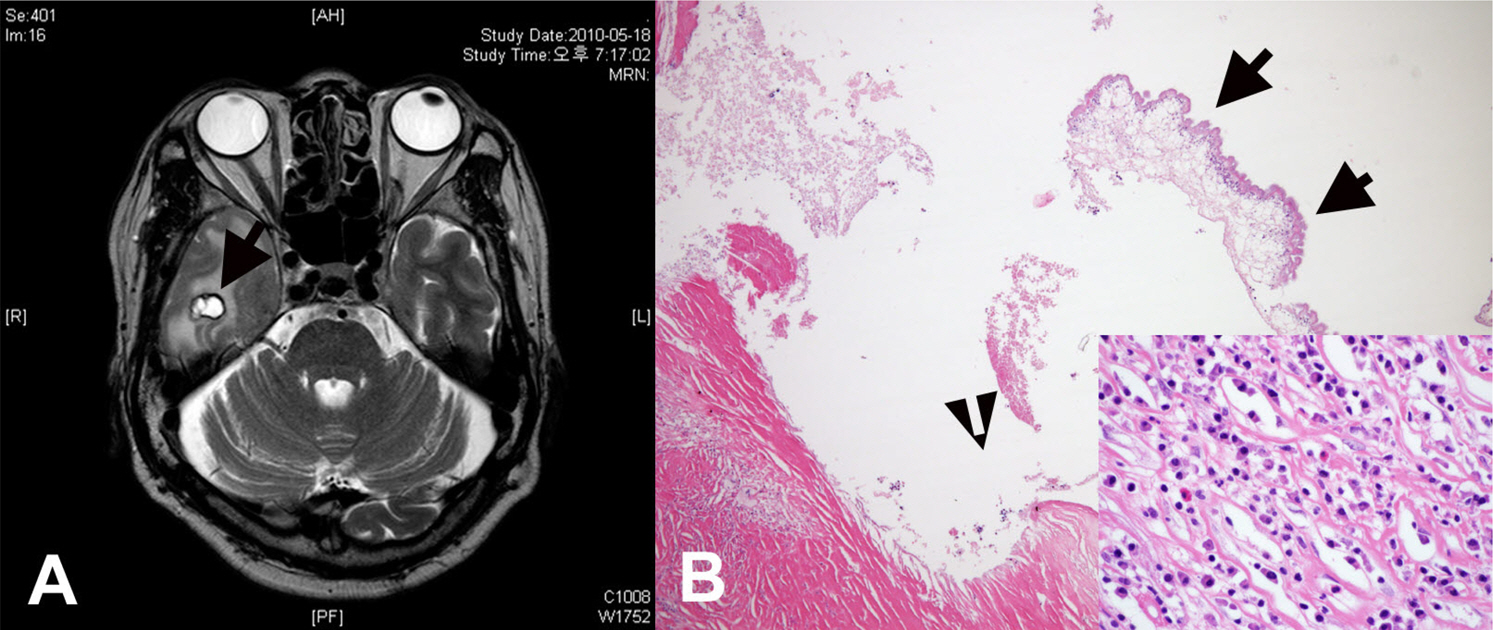
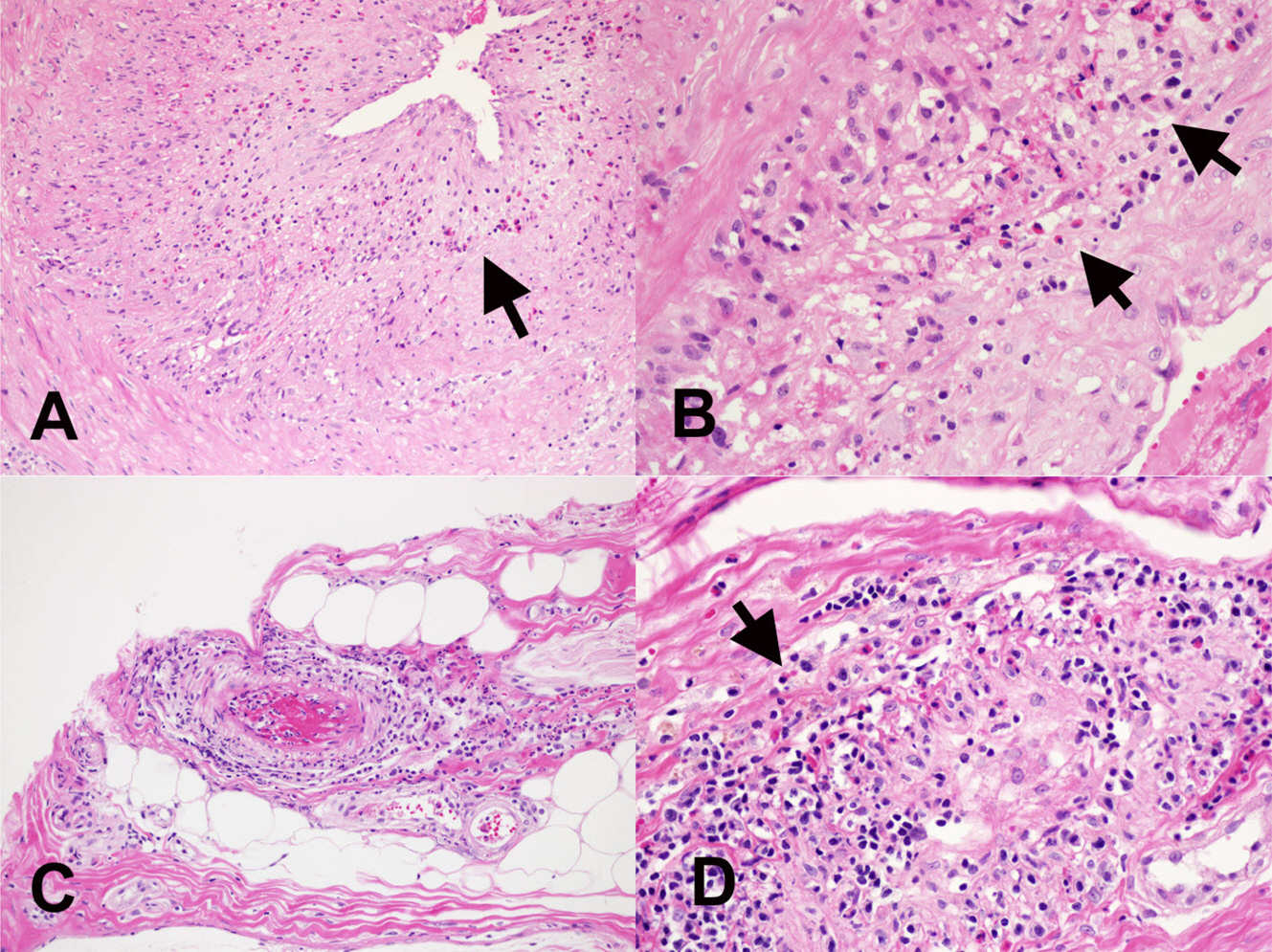
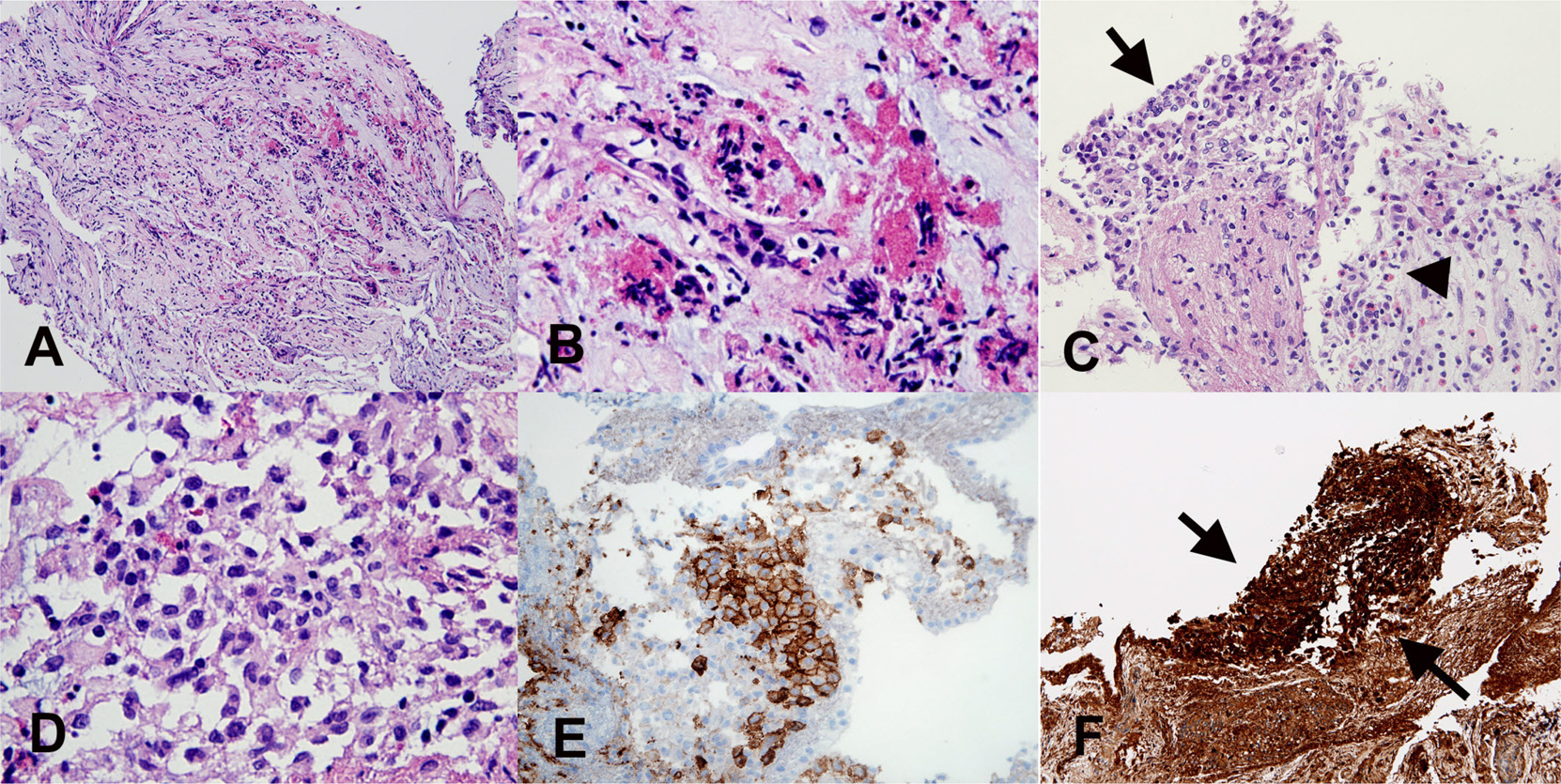
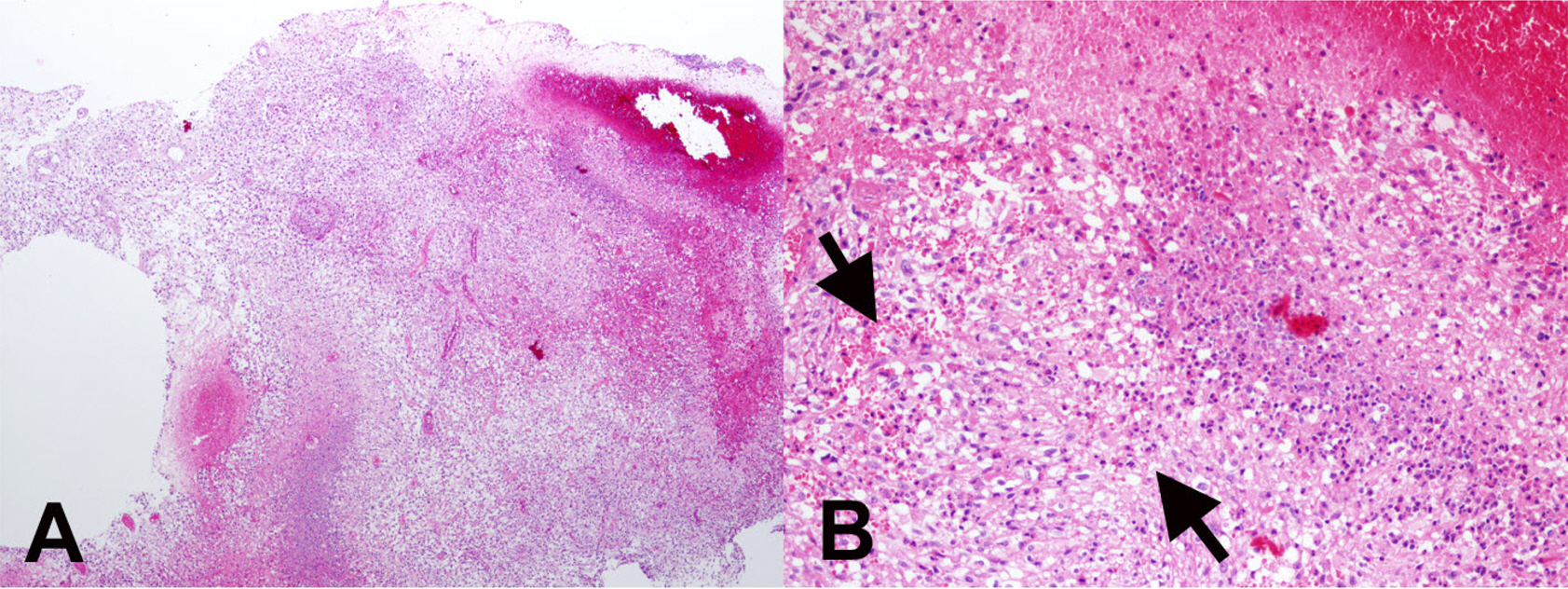
- Eosinophils are one of the polymorphonuclear granulocytes derived from bone marrow stem cells, and they contain many small cytoplasmic granules that stain bright red with eosin or brick-red with Romanowsky staining. Eosinophilic infiltration is also present in various human central nervous system (CNS) diseases such as parasitic infection, transverse myelitis, vasculitis, Langerhans cell histiocytosis, glioblastoma and etc... Due to the morphologic and functional characteristics, the presence of eosinophils in certain lesions may provide useful diagnostic clues in the right clinical setting. Consideration of this finding may facilitate the diagnosis of CNS pathologic lesions, especially in a small specimen such as a stereotactic biopsy.
Keyword
MeSH Terms
Figure
Reference
-
1.Young B. Wheater's functional histology: a text and colour atlas. 5th ed.Edinburgh: Churchill Livingstone/Elsevier;2006.2.Curran CS., Bertics PJ. Eosinophils in glioblastoma biology. J Neuroinflammation. 2012. 9:11.
Article3.Seehusen DA., Reeves MM., Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003. 68:1103–8.4.Ramzy I. Clinical cytopathology and aspiration biopsy: Fundamental principles and practice. 2nd ed.New York: McGraw-Hill;2001.5.Luna LG. The quality control dilemma in Histotechnology: A possible answer. Histologic. 1991. 21:245–50.6.Goffette S., Jeanjean AP., Duprez TP., Bigaignon G., Sindic CJ. Eosinophilic pleocytosis and myelitis related to Toxocara canis infection. Eur J Neurol. 2000. 7:703–6.7.Harzheim M., Schlegel U., Urbach H., Klockgether T., Schmidt S. Discriminatory features of acute transverse myelitis: a retrospective analysis of 45 patients. J Neurol Sci. 2004. 217:217–23.
Article8.Jeffery DR., Mandler RN., Davis LE. Transverse myelitis. Retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch Neurol. 1993. 50:532–5.9.Kawamura J., Kohri Y., Oka N. Eosinophilic meningoradiculo- myelitis caused by Gnathostoma spinigerum. A case report. Arch Neurol. 1983. 40:583–5.10.Kerr DA., Ayetey H. Immunopathogenesis of acute transverse myelitis. Curr Opin Neurol. 2002. 15:339–47.
Article11.Kikuchi H., Osoegawa M., Ochi H., Murai H., Horiuchi I., Takahashi H, et al. Spinal cord lesions of myelitis with hyperIgEemia and mite antigen specific IgE (atopic myelitis) manifest eosinophilic inflammation. J Neurol Sci. 2001. 183:73–8.
Article12.Tsai CP., Yeh HH., Tsai JJ., Lin KP., Wu ZA. Transverse myelitis and polyneuropathy in idiopathic hypereosinophilic syndrome. Muscle Nerve. 1993. 16:112–3.13.Kira J., Yamasaki K., Kawano Y., Kobayashi T. Acute myelitis associated with hyperIgEemia and atopic dermatitis. J Neurol Sci. 1997. 148:199–203.
Article14.Kira J., Kawano Y., Yamasaki K., Tobimatsu S. Acute myelitis with hyperIgEaemia and mite antigen specific IgE: atopic myelitis. J Neurol Neurosurg Psychiatry. 1998. 64:676–9.
Article15.Osoegawa M., Ochi H., Kikuchi H., Shirabe S., Nagashima T., Tsumoto T, et al. Eosinophilic myelitis associated with atopic diathesis: a combined neuroimaging and histopathological study. Acta Neuropathol (Berl). 2003. 105:289–95.
Article16.Osoegawa M., Ochi H., Minohara M., Murai H., Umehara F., Furuya H, et al. Myelitis with atopic diathesis: a nationwide survey of 79 cases in Japan. J Neurol Sci. 2003. 209:5–11.
Article17.Lucchinetti CF., Mandler RN., McGavern D., Bruck W., Gleich G., Ransohoff RM, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002. 125:1450–61.18.Milici AJ., Carroll LA., Stukenbrok HA., Shay AK., Gladue RP., Showell HJ. Early eosinophil infiltration into the optic nerve of mice with experimental allergic encephalomyelitis. Lab Invest. 1998. 78:1239–44.19.Giannini C., Salvarani C., Hunder G., Brown RD. Primary central nervous system vasculitis: pathology and mechanisms. Acta Neuropathol. 2012. 123:759–72.
Article20.Nordborg E., Nordborg C. Giant cell arteritis. Kalimo H, editor. editor.Pathology & Genetics Cerebrovascular Diseases. Basel: International Society of Neuropathology;2005. p. 134–9.21.Bazile C., Keohane C., Gray F. Systemic Diseases and Drug-Induced Vasculitis. Kalimo H, editor. editor.Pathology & Genetics Cerebrovascular Diseases. Basel: International Society of Neuropathology;2005. p. 151–62.22.Guillevin L., Cohen P., Gayraud M., Lhote F., Jarrousse B., Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine. 1999. 78:26–37.
Article23.Na SJ., Lee KO., Ko JH. Eosinophilic vasculitis of the spinal cord associated with Churg-Strauss syndrome. J Neurol Sci. 2010. 295:107–9.
Article24.Jennette JC., Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997. 337:1512–23.
Article25.Kono H., Inokuma S., Nakayama H., Yamazaki J. Pachymeningitis in microscopic polyangiitis (MPA): a case report and a review of central nervous system involvement in MPA. Clin Exp Rheumatol. 2000. 18:397–400.26.Furukawa Y., Matsumoto Y., Yamada M. Hypertrophic pachymeningitis as an initial and cardinal manifestation of microscopic polyangiitis. Neurology. 2004. 63:1722–4.
Article27.Huang CH., Hung CH., Chu YT., Hua YM. Tumor-like cerebral perivasculitis in a pediatric patient with systemic lupus erythematosus. Kaohsiung J Med Sci. 2008. 24:218–22.
Article28.Moezzi J., Gopalswamy N., Haas RJ Jr., Markert RJ., Suryaprasad S., Bhutani MS. Stromal eosinophilia in colonic epithelial neoplasms. Am J Gastroenterol. 2000. 95:520–3.
Article29.Kural YB., Su O., Onsun N., Uras AR. Atopy, IgE and eosinophilic cationic protein concentration, specific IgE positivity, eosinophil count in cutaneous T Cell lymphoma. Int J Dermatol. 2010. 49:390–5.
Article30.Iwasaki K., Torisu M., Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986. 58:1321–7.
Article31.Dorta RG., Landman G., Kowalski LP., Lauris JR., Latorre MR., Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002. 41:152–7.
Article32.Moroni M., Porta C., De Amici M., Quaglini S., Cattabiani MA., Buzio C. Eosinophils and C4 predict clinical failure of combination immunotherapy with very low dose subcutaneous interleukin-2 and interferon in renal cell carcinoma patients. Haematologica. 2000. 85:298–303.33.Cormier SA., Taranova AG., Bedient C., Nguyen T., Protheroe C., Pero R, et al. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006. 79:1131–9.
Article34.Sherman PW., Holland E., Sherman JS. Allergies: their role in cancer prevention. Q Rev Biol. 2008. 83:339–62.
Article35.Mueller MM., Herold-Mende CC., Riede D., Lange M., Steiner HH., Fusenig NE. Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol. 1999. 155:1557–67.
Article36.Curran CS., Evans MD., Bertics PJ. GM-CSF production by glioblastoma cells has a functional role in eosinophil survival, activation, and growth factor production for enhanced tumor cell proliferation. J Immunol. 2011. 187:1254–63.
Article37.Smith D., Shimamura T., Barbera S., Bejcek BE. NF-kappaB controls growth of glioblastomas/astrocytomas. Mol Cell Biochem. 2008. 307:141–7.38.Ranza E., Facoetti A., Morbini P., Benericetti E., Nano R. Exogenous platelet derived growth factor (PDGF) induces human astrocytoma cell line proliferation. Anticancer Res. 2007. 27:2161–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Time Course of Remote Neuropathology Following Diffuse Traumatic Brain Injury in the Male Rat
- Neuropathologic features of central nervous system hemangioblastoma
- Recurrence after Modified Mini-Flap Technique for Pterygium Surgery
- Dermatological Signs of Systemic Diseases
- Validity of Dementia Screening Test 'Korean Version of the Mini-Cog'