Transl Clin Pharmacol.
2016 Jun;24(2):78-83. 10.12793/tcp.2016.24.2.78.
Evaluation of factors associated with drug-induced liver injury using electronic medical records
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 03080, Korea.
- 2Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Bundang Hospital, Seongnam 13620, Korea. jychung@snubh.org
- KMID: 2290473
- DOI: http://doi.org/10.12793/tcp.2016.24.2.78
Abstract
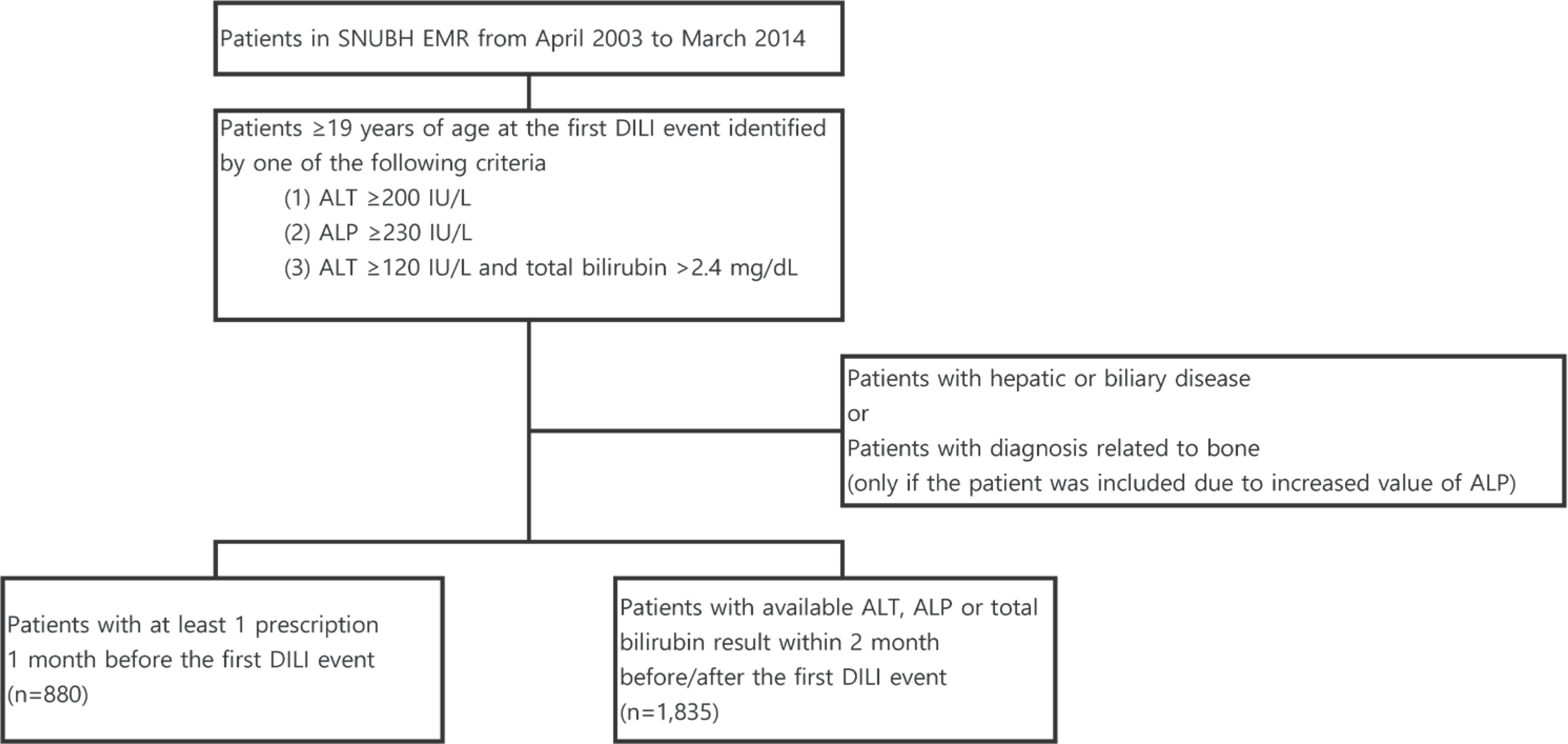
- The causes and attributing factors of drug-induced liver injury (DILI) remain unclear as a result of exclusion-based diagnosis and low incidence. The aim of this study was to explore and evaluate potential drug-related causes and factors associated with DILI. Using electronic medical records (EMR) from the Seoul National University Bundang Hospital from 2003 to 2014, patients with DILI events were identified based on liver function test results. All patients with hepatic or biliary diseases were excluded. Patient characteristics, including demographics, clinical patterns, and severity of DILI were summarized and their associations were evaluated. Drugs frequently prescribed to patients exhibiting DILI within the month before their first DILI event compared to the total patient population were identified and the probabilities of hepatotoxicity associated with their use were assessed through examination of available reports. Among the 1,835 patients with laboratory test results, 1,023 were male and 1,053 were 65 years of age or older. Moderate DILI was dominant in older or male patients and cholestatic DILI tended to be more frequently identified in older patients of either sex. Cytarabine was the most frequently prescribed drug in DILI patients, followed by aprotinin and dopamine. Among the 30 most frequently prescribed drugs in DILI patients, 15 (50%) were identified as known hepatotoxic agents. In conclusion, this study evaluated differences in features of DILI among groups based on demographics and explored candidate drugs with possible associations with DILI, which has potential value reflecting real-world clinical practice.
MeSH Terms
Figure
Reference
-
References
1. Teschke R, Frenzel C, Wolff A, Eickhoff A, Schulze J. Drug induced liver injury: accuracy of diagnosis in published reports. Ann Hepatol. 2014; 13:248–255.
Article2. Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002; 36:451–455. doi: 10.1053/jhep.2002.34857.
Article3. Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013; 144:1419–1425. 1425 e1411-1413.doi: 10.1053/j.gastro.2013.02.006.
Article4. Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011; 89:806–815. doi: 10.1038/clpt.2011.58.
Article5. Shin J, Hunt CM, Suzuki A, Papay JI, Beach KJ, Cheetham TC. Characterizing phenotypes and outcomes of drug-associated liver injury using electronic medical record data. Pharmacoepidemiol Drug Saf. 2013; 22:190–198. doi: 10.1002/pds.3388.
Article6. Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990; 11:272–276.7. Micromedex® 2.0 (electronic version). http://www.micromedexsolutions. com. Accessed Mar 20. 2016.8. Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005; 129:512–521. doi: 10.1016/j.gastro.2005.05.006.
Article9. Lucena MI, Andrade RJ, Kaplowitz N, Garcia-Cortes M, Fernandez MC, Romero-Gomez M, et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009; 49:2001–2009. doi: 10.1002/hep.22895.
Article10. Herzig RH, Wolff SN, Lazarus HM, Phillips GL, Karanes C, Herzig GP. High-dose cytosine arabinoside therapy for refractory leukemia. Blood. 1983; 62:361–369.
Article11. Suzuki A, Andrade RJ, Bjornsson E, Lucena MI, Lee WM, Yuen NA, et al. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase: unified list based on international collaborative work. Drug Saf. 2010; 33:503–522. doi: 10.2165/11535340-000000000-00000.12. Kagawa T, Hirose S, Arase Y, Oka A, Anzai K, Tsuruya K, et al. No contribution of the ABCB11 p.444A polymorphism in Japanese patients with drug-induced cholestasis. Drug Metab Dispos. 2015; 43:691–697. doi: 10.1124/dmd.114.061325.
Article13. Dauby N, Fink W, Seyler L, Luce S, Nouwynck C, Tas S, et al. Probable hypersensitivity reaction to vancomycin associating rash, fever and neutropenia. Acta Clin Belg. 2012; 67:226–228. doi: 10.2143/ACB.67.3.20626 62.14. Song SM, Cho MS, Oh SH, Kim KM, Park YS, Kim DY, et al. Liver transplantation in a child with acute liver failure resulting from drug rash with eosinophilia and systemic symptoms syndrome. Korean J Pediatr. 2013; 56:224–226. doi: 10.3345/kjp.2013.56.5.224.
Article15. LiverTox. LiverTox.nih.gov. Accessed Mar 30. 2016.16. Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012; 107:1380–1387. doi: 10.1038/ajg.2012.138.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Active Pharmacovigilance of Drug-Induced Liver Injury Using Electronic Health Records
- Drug Induced Liver Injury by Prophylactic Administration of Albendazole
- Factors affecting drug-induced liver injury: antithyroid drugs as instances
- Do Natural Health Products Cause Toxic Hepatitis?
- Evaluation of Drug-Induced Liver Injury Developed During Hospitalization Using Electronic Health Record (EHR)-Based Algorithm