J Clin Neurol.
2006 Mar;2(1):1-11. 10.3988/jcn.2006.2.1.1.
Small Vessel Disease and Subcortical Vascular Dementia
- Affiliations
-
- 1Wolfson Research Centre, Institute for Ageing and Health, Newcastle General Hospital, Westgate Road, Newcastle upon Tyne NE4 6BE, United Kingdom. r.n.klaria@ncl.ac.uk
- 2Memory Research Unit, Department of Neurology, Helsinki University Central Hospital, PO Box 300, 00290 HYKS, Helsinki, Finland.
- KMID: 2287698
- DOI: http://doi.org/10.3988/jcn.2006.2.1.1
Abstract
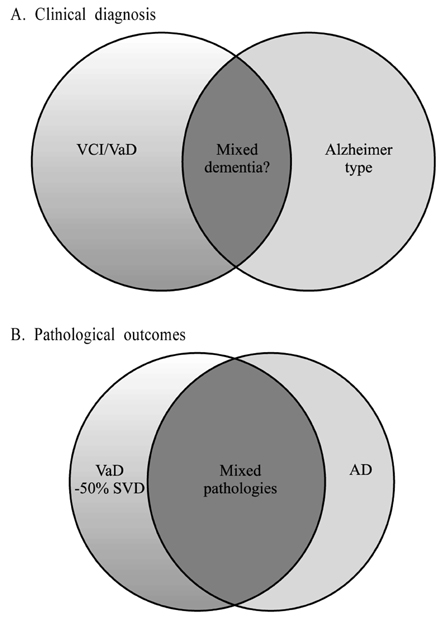
- Atherothromboembolism and intracranial small vessel disease are considered to be the main causes of cerebrovascular injury, which may lead to cognitive impairment and vascular dementia (VaD). VaD appears to be the second most common type of dementia with prevalence estimates of 10-15%. Cortical or multi-infarct dementia and subcortical vascular dementia (SVD) are suggested to be the two main forms of VaD. The main clinical features of SVD comprise decreased motor performance, early impairment of attention and executive function with slowing of information processing. SVD results from lacunar infarcts or multiple microinfarcts in the basal ganglia, thalamus, brainstem and white matter and are associated with more than 50% of the VaD cases. White matter changes including regions of incomplete infarction are usually widespread in VaD but their contribution to impairment of subcortical regions is unclear. While most of VaD occurs sporadically only a small proportion of cases bear clear familial traits. CADASIL is likely the most common form of hereditary VaD, which arises from subcortical arteriopathy. SVD needs unambiguous definition to impact on preventative and treatment strategies, and critical for selective recruitment to clinical trials.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Total Cerebral Small-Vessel Disease Score is Associated with Mortality during Follow-Up after Acute Ischemic Stroke
Tae-Jin Song, Jinkwon Kim, Dongbeom Song, Joonsang Yoo, Hye Sun Lee, Yong-Jae Kim, Hyo Suk Nam, Ji Hoe Heo, Young Dae Kim
J Clin Neurol. 2017;13(2):187-195. doi: 10.3988/jcn.2017.13.2.187.
Reference
-
1. Berrios GE, Freeman HL. Alzheimer and the dementias. 1991. London: Royal Society of Medicine Services;69–76. Eponymists in Medcine series.2. O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, et al. Vascular cognitive impairment. Lancet Neurol. 2003. 2:89–98.3. Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002. 1:426–436.
Article4. Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular Dementia: Diagnostic Criteria for Research Studies. Report of the NINDS-AIREN International Work Group. Neurology. 1993. 43:250–260.
Article5. Erkinjuntti T. Clinical criteria for vascular dementia: The NINDS-AIREN criteria. Dementia. 1994. 5:189–192.
Article6. Rockwood K, Parhad I, Hachinski V, Erkinjuntti T, Rewcastle B, Kertesz A, et al. Diagnosis of vascular dementia: Consortium of Canadian Centres for Clinical Cognitive Research consensus statement. Can J Neurol Sci. 1994. 21:358–364.
Article7. Wetterling T, Kanitz RD, Borgis KJ. Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD-10, NINDS-AIREN). Stroke. 1996. 27:30–36.
Article8. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 1994. 4 ed. Washington, DC: American Psychiatric Association.9. World Health Organization. ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for research. 1993. Geneva: WHO.10. Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischaemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992. 42:473–480.
Article11. Erkinjuntti T, Ostbye T, Steenhuis R, Hachinski V. The effect of different diagnostic criteria on the prevalence of dementia. N Engl J Med. 1997. 337:1667–1674.
Article12. Wetterling T, Kanitz RD, Borgis KJ. The ICD-10 criteria for vascular dementia. Dementia. 1994. 5:185–188.
Article13. Erkinjuntti T, Bowler JV, DeCarli C, Fazekas F, Inzitari D, O'Brien JT, et al. Imaging of static brain lesions in vascular dementia: implications for clinical trials. Alzheimer Dis Assoc Disord. 1999. 13 Suppl 3:S81–S90.
Article14. Pohjasvaara T, Erkinjuntti T, Vataja R, Kaste M. Dementia three months after stroke. Baseline frequency and effect of different definitions of dementia in the Helsinki Stroke Aging Memory Study (SAM) cohort. Stroke. 1997. 28:785–779.15. Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med. 1993. 328:153–158.
Article16. Gold G, Giannakopoulos P, Montes-Paixao JC, Herrmann FR, Mulligan R, Michel JP, et al. Sensitivity and specificity of newly proposed clinical criteria for possible vascular dementia. Neurology. 1997. 49:690–694.
Article17. Jellinger KA. Vascular-ischemic dementia: an update. J Neural Transm Suppl. 2002. 62:1–23.
Article18. Markesbery W. Markesbery WR, editor. Vascular dementia. Neuropathology of dementing disorders. 1998. London: Arnold;293–311.19. Seno H, Ishino H, Inagaki T, Iijima M, Kaku K, Inata T. A neuropathological study of dementia in nursing homes over a 17-year period in Shimane Prefecture, Japan. Gerontology. 1999. 45:44–48.
Article20. Akatsu H, Takahashi M, Matsukawa N, Ishikawa Y, Kondo N, Sato T, et al. Subtype analysis of neuropathologically diagnosed patients in a Japanese geriatric hospital. J Neurol Sci. 2002. 196:63–69.
Article21. Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999. 354:919–920.
Article22. Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiat. 1997. 63:749–753.
Article23. Rossi R, Joachim C, Geroldi C, Combrinck M, Esiri MM, Smith AD, et al. Association between subcortical vascular disease on CT and neuropathological findings. Int J Geriatr Psychiatry. 2004. 19:690–695.
Article24. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997. 277:813–817.
Article25. Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer's disease. Am J Pathol. 1996. 148:2083–2095.26. Heyman A, Fillenbaum GG, Welsh-Bohmer KA, Gearing M, Mirra SS, Mohs RC, Peterson BL, Pieper CF. Cerebral infarcts in patients with autopsy-proven Alzheimer's disease. CERAD Part XVIII. Neurology. 1998. 51:159–162.
Article27. Heyman A, Fillenbaum GG, Welsh-Bohmer KA, Gearing M, Mirra SS, Mohs RC, et al. Comparison of pathological diagnostic criteria for Alzheimer disease. Alzheimer Dis Assoc Disord. 1998. 12:182–189.
Article28. Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999. 13:S115–S123.
Article29. Erkinjuntti T, Haltia M, Palo J, Sulkava R, Paetau A. Accuracy of the clinical diagnosis of vascular dementia: a prospective clinical and post-mortem neuropathological study. J Neurol Neurosurg Psychiatry. 1988. 51:1037–1044.
Article30. Hulette C, Nochlin D, McKeel D, Morris JC, Mirra SS, Sumi SM, et al. Clinical-neuropathologic findings in multi-infarct dementia: a report of six autopsied cases. Neurology. 1997. 48:668–672.
Article31. Nolan KA, Lino MM, Seligmann AW, Blass JP. Absence of vascular dementia in an autopsy series from a dementia clinic. J Am Geriatr Soc. 1998. 46:597–604.
Article32. Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980. 7:486–488.
Article33. Gold G, Bouras C, Canuto A, Bergallo MF, Herrmann FR, Hof PR, et al. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry. 2002. 159:82–87.
Article34. Ballard C, McKeith I, O'Brien J, Kalaria R, Jaros E, Ince P, et al. Neuropathological substrates of dementia and depression in vascular dementia, with a particular focus on cases with small infarct volumes. Dement Geriatr Cogn Disord. 2000. 11:59–65.
Article35. Kalaria RN, Ballard CG. Stroke and Cognition. Curr Atheroscler Rep. 2001. 3:334–339.
Article36. Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002. 359:1283–1290.
Article37. Kalimo H, Kaste M, Haltia M. Graham D, Lantos P, editors. Vascular diseases. 2002a. Greenfield's Neuropathology Arnold Press;281–255.38. Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, et al. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000. 59:931–945.
Article39. Brun A. Pathology and pathophysiology of cerebrovascular dementia: pure subgroups of obstructive and hypoperfusive etiology. Dementia. 1994. 5:145–147.
Article40. Cummings JL. Vascular subcortical dementias: clinical aspects. Dementia. 1994. 5:177–180.
Article41. Wallin A, Blennow K. The clinical diagnosis of vascular dementia. Dementia. 1994. 5:181–184.
Article42. Wallin A, Milos V, Sjögren M, Pantoni L, Erkinjuntti T. Classification and subtypes of vascular dementia. Int Psychogeriatr. 2003. 15:27–37.
Article43. Sulkava R, Erkinjuntti T. Vascular dementia due to cardiac arrhythmias and systemic hypotension. Acta Neurol Scand. 1987. 76:123–128.
Article44. Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl. 2000. 59:23–30.
Article45. Babikian V, Ropper AH. Binswanger's disease: a review. Stroke. 1987. 18:2–12.
Article46. Ishii N, Nishihara Y, Imamura T. Why do frontal lobe symptoms predominate in vascular dementia with lacunes? Neurology. 1986. 36:340–345.
Article47. Mahler ME, Cummings JL. The behavioural neurology of multi-infarct dementia. Alzheimer Dis Assoc Disord. 1991. 5:122–130.48. Roman G.C. Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA. 1987. 258:1782–1788.
Article49. Wallin A, Blennow K, Gottfries CG. Subcortical symptoms predominate in vascular dementia. Int J Geriatr Psychiatry. 1991. 6:137–146.
Article50. Erkinjuntti T. Types of multi-infarct dementia. Acta Neurol Scand. 1987. 75:391–399.
Article51. Fischer P, Gatterer G, Marterer A, Simanyi M, Danielczyk W. Course characteristics in the differentiation of dementia of the Alzheimer type and multi-infarct dementia. Acta Psychiatr Scand. 1990. 81:551–553.
Article52. Skoog I. Hansson L, Birkenhäger WH, editors. Blood pressure and dementia. Handbook of hypertension. 1997. Vol 18. Amsterdam: Elsevier Science BV;303–331. Assessment of hypertensive organ damage.53. Desmond DW, Erkinjuntti T, Sano M, Cummings JL, Bowler JV, Pasquier F, et al. The cognitive syndrome of vascular dementia: implications for clinical trials. Alzheimer Dis Assoc Disord. 1999. 13 Suppl 3:S21–S29.
Article54. Pillon B, Deweer B, Agid Y, Dubois B. Explicit memory in Alzheimer's, Huntington's, and Parkinson's diseases. Arch Neurol. 1993. 50:374–379.
Article55. Kertesz A, Clydesdale S. Neuropsychological deficits in vascular dementia vs Alzheimer's disease. Frontal lobe deficits prominent in vascular dementia. Arch Neurol. 1994. 51:1226–1231.
Article56. Traykov L, Baudic S, Thibaudet MC, Rigaud AS, Smagghe A, Boller F. Neuropsychological deficit in early subcortical vascular dementia: comparison to Alzheimer's disease. Dement Geriatr Cogn Disord. 2002. 14:26–32.
Article57. Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JCL, Wen W, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004. 62:912–919.
Article58. Cummings JL. Fronto-subcortical circuits and human behavior. Arch Neurol. 1993. 50:873–880.59. Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci. 2004. 226:75–80.
Article60. Lammie G. Pathology of small vessel stroke. Br Med Bull. 2000. 56:296–306.
Article61. Ho K-L, Garcia JH. Pantoni L, Inzitari D, Wallin A, editors. Neuropathology of the small blood vessels in selected disease of the cerebral white matter. The matter of white matter. 2000. 10. Utrecht, The Netherlands: Academic Pharmaceutical Productions;247–273. Current Issues in Neurodegenerative diseases.62. Kalaria RN, Hedera P. Differential degeneration of the endothelium and basement membrane of capillaries in Alzheimer's disease. Neuroreport. 1995. 6:477–480.
Article63. Benhaiem-Sigaux N, Gray F, Gherardi R, Roucayrol AM, Poirier J. Expanding cerebellar lacunes due to dilatation of the perivascular space associated with Binswanger's subcortical arteriosclerotic encephalopathy. Stroke. 1987. 18:1087–1092.
Article64. White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia aging study participants. Ann NY Acad Sci. 2002. 977:9–23.
Article65. Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, et al. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004. 35:410–414.
Article66. Suter OC, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002. 33:1986–1992.
Article67. Kalaria RN, Thomas A, Oakley A, Ince P, Tamaoka A, Mori H, et al. Cerebrovascular amyloidosis and dementia. Curr Med Chem Immun Endoc & Metab Agents. 2003. 4:317–327.
Article68. Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987. 18:311–324.
Article69. Greenberg SM. Cerebral amyloid angiopathy and vessel dysfunction. Cerebrovasc Dis. 2002. 13:42–47.
Article70. Kalaria RN. Advances in molecular genetics and pathology of cerebrovascular disorders. Trends Neurosci. 2001. 24:392–400.
Article71. Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004. 35:2616–2619.
Article72. Haan J, Lanser JBK, Zijderveld I, van der Does IGF, Roos RAC. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type. Arch Neurol. 1990. 47:965–967.
Article73. Bornebroek M, Haan J, Roos RA. Hereditary cerebral hemorrhage with amyloidosis--Dutch type (HCHWA-D): a review of the variety in phenotypic expression. Amyloid. 1999. 6:215–224.
Article74. Natte R, Maat-Schieman ML, Haan J, Bornebroek M, Roos RA, van Duinen SG. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type is associated with cerebral amyloid angiopathy but is independent of plaques and neurofibrillary tangles. Ann Neurol. 2001. 50:765–772.
Article75. Haglund M, Englund E. Cerebral amyloid angiopathy, white matter lesions and Alzheimer encephalopathy - a histopathological assessment. Dement Geriatr Cogn Disord. 2002. 14:161–166.
Article76. Sarazin M, Amarenco P, Mikol J, Dimitri D, Lot G, Bousser MG. Reversible leukoencephalopathy in cerebral amyloid angiopathy presenting as subacute dementia. Eur J Neurol. 2002. 9:353–358.
Article77. Kalimo H, Ruchoux MM, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol. 2002. 12:371–384.
Article78. Chabriat H, Vahedi K, Iba-Zizen MT, Joutel A, Nibbio A, Nagy TG, et al. Clinical spectrum of CADASIL: a study of 7 families. Lancet. 1995. 346:934–939.
Article79. Dichgans M, Mayer M, Uttner I, Bruning R, Muller-Hocker J, Rungger G, et al. The phenotypic spectrum of CADASIL: Clinical findings in 102 cases. Ann Neurol. 1998. 44:731–739.
Article80. Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004. 127:2533–2539.
Article81. Singhal S, Bevan S, Barrick T, Rich P, Markus HS. The influence of genetic and cardiovascular risk factors on the CADASIL phenotype. Brain. 2004. 127:2031–2038.
Article82. Chabriat H, Levy C, Taillia H, Iba-Zizen MT, Vahedi K, Joutel A, et al. Patterns of MRI lesions in CADASIL. Neurology. 1998. 51:452–457.
Article83. Lesnik Oberstein SA, van den Boom R, van Buchem MA, van Houwelingen HC, Bakker E, Vollebregt E, et al. The Dutch CADASIL Research Group. Cerebral microbleeds in CADASIL. Neurology. 2001. 57:1066–1070.84. Ruchoux MM, Maurage CA. CADASIL: Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy. J Neuropathol Exp Neurol. 1997. 56:947–964.85. Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997. 350:1511–1515.
Article