J Breast Cancer.
2012 Mar;15(1):15-23. 10.4048/jbc.2012.15.1.15.
Aberrant Expression of Cancer Stem Cells Marker Prominin-1 in Low-Grade Tubulobular Breast Carcinoma: A Correlative Study between qRT-PCR, Flow-Cytometric and Immunohistochemistry Analysis
- Affiliations
-
- 1Pathology Unit, National Cancer Institute, Pascale Hospital, Naples, Italy. monicantile@libero.it
- 2Department of Experimental Oncology, National Cancer Institute, Pascale Hospital, Naples, Italy.
- KMID: 2286469
- DOI: http://doi.org/10.4048/jbc.2012.15.1.15
Abstract
- PURPOSE
Prominin1/CD133 has become the ideal marker for cancer stem cells (CSCs) detection in human tumors. In this study we examined the expression of this marker in several breast cancer specimens to associate CSCs percentage with risk factor for this neoplasia.
METHODS
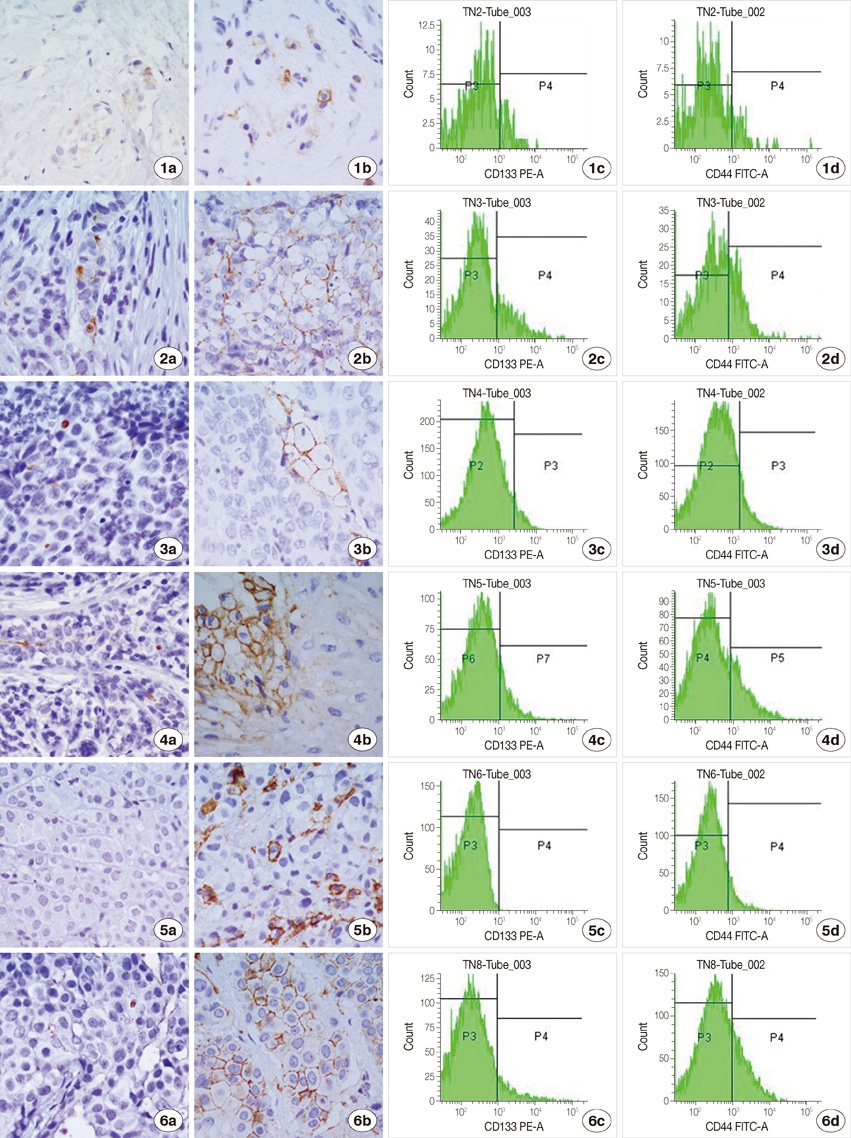
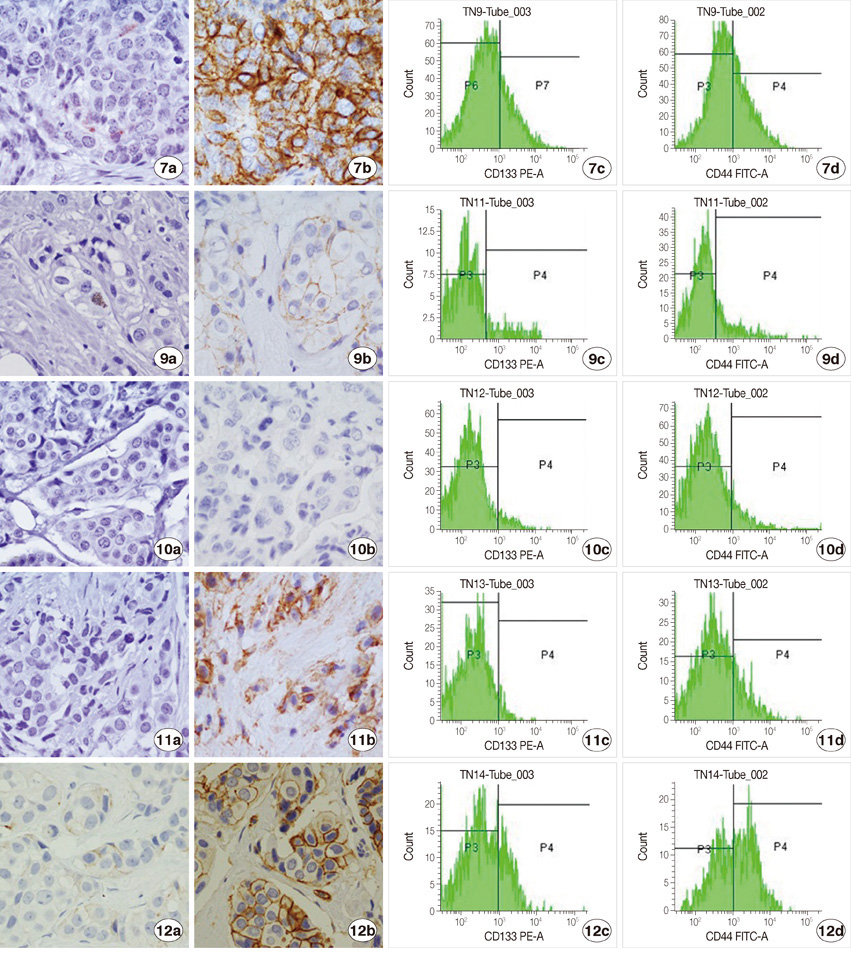
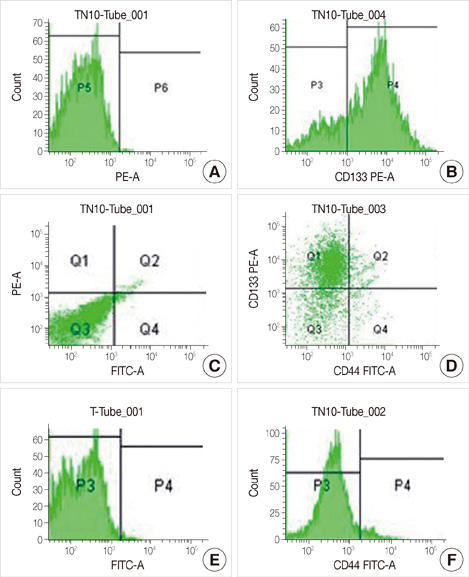
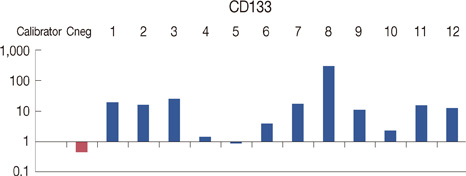
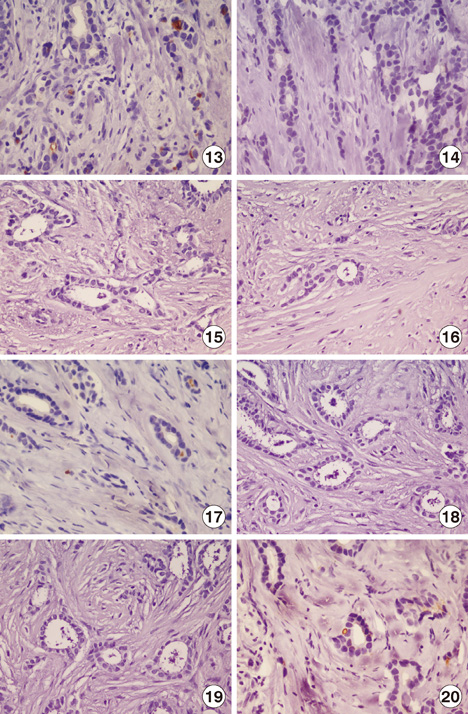
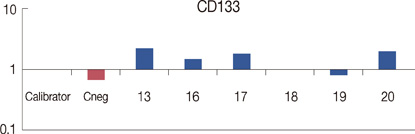
We examined specimens from 12 patients using CD133 and CD44 antibodies for CSCs immunohistochemistry detection and for flow cytometry analysis. For each patient, we also performed the immunohistochemical staining to evaluate the expression of estrogen receptor, progesterone receptor, c-erbB-2, Ki67, and E-cadherin markers. A Taqman probe for CD133 was used for mRNA quantification by real-time polymerase chain reaction.
RESULTS
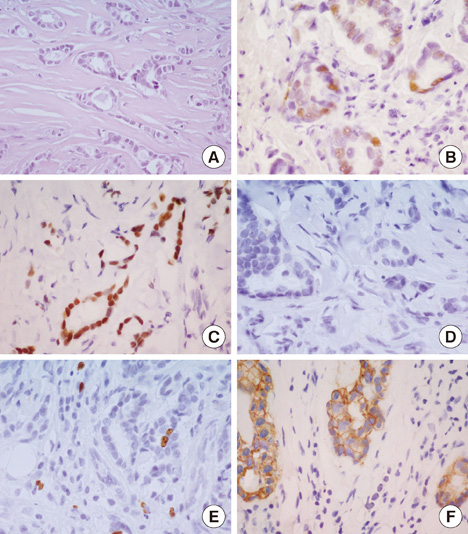
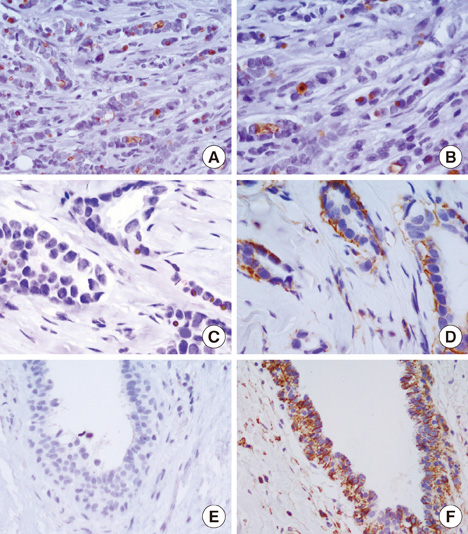
Prominin-1 expression was heterogeneous in different carcinomas but was strikingly hyperexpressed in a tubulolobular variant of breast cancer. The results were confirmed by all three methods.
CONCLUSION
Our data, although produced on a limited number of samples, showed an particularly high expression of stem cell marker CD133 in a breast cancer variant, generally with a good prognosis. Since CSCs detection by CD133 has been described as an important prognostic factor for several human cancers, we suggest the importance of detecting stem cell compartiments in all histotypes of breast carcinomas.
MeSH Terms
-
Antibodies
Antigens, CD
Breast
Breast Neoplasms
Cadherins
Estrogens
Flow Cytometry
Glycoproteins
Humans
Immunohistochemistry
Neoplastic Stem Cells
Peptides
Prognosis
Receptors, Progesterone
Risk Factors
RNA, Messenger
Stem Cells
Antibodies
Antigens, CD
Cadherins
Estrogens
Glycoproteins
Peptides
RNA, Messenger
Receptors, Progesterone
Figure
Cited by 1 articles
-
Prominin-1 and Its Role in Tumor Progression and Assessment of Clinical Prognosis in Systemic Malignancies
Shailendra Kapoor
J Breast Cancer. 2013;16(2):244-244. doi: 10.4048/jbc.2013.16.2.244.
Reference
-
1. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006. 355:1253–1261.
Article2. Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007. 18:460–466.
Article3. Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004. 23:7274–7282.
Article4. Maitland NJ, Collins AT. Cancer stem cells: a therapeutic target? Curr Opin Mol Ther. 2010. 12:662–673.5. Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009. 106:13820–13825.
Article6. Kim HJ, Kim JB, Lee KM, Shin I, Han W, Ko E, et al. Isolation of CD24(high) and CD24(low/-) cells from MCF-7: CD24 expression is positively related with proliferation, adhesion and invasion in MCF-7. Cancer Lett. 2007. 258:98–108.
Article7. Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006. 8:R59.8. Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008. 10:R53.9. Rappa G, Lorico A. Phenotypic characterization of mammosphere-forming cells from the human MA-11 breast carcinoma cell line. Exp Cell Res. 2010. 316:1576–1586.
Article10. Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997. 90:5013–5021.
Article11. Fargeas CA, Corbeil D, Huttner WB. AC133 antigen, CD133, prominin-1, prominin-2, etc.: prominin family gene products in need of a rational nomenclature. Stem Cells. 2003. 21:506–508.
Article12. Wu Y, Wu PY. CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev. 2009. 18:1127–1134.
Article13. Liu Q, Li JG, Zheng XY, Jin F, Dong HT. Expression of CD133, PAX2, ESA, and GPR30 in invasive ductal breast carcinomas. Chin Med J (Engl). 2009. 122:2763–2769.14. Zhao P, Lu Y, Jiang X, Li X. Clinicopathological significance and prognostic value of CD133 expression in triple-negative breast carcinoma. Cancer Sci. 2011. 102:1107–1111.
Article15. Lorico A, Rappa G. Phenotypic heterogeneity of breast cancer stem cells. J Oncol. 2011. 2011:135039.
Article16. Al-Mulla F, Abdulrahman M, Varadharaj G, Akhter N, Anim JT. BRCA1 gene expression in breast cancer: a correlative study between real-time RT-PCR and immunohistochemistry. J Histochem Cytochem. 2005. 53:621–629.
Article17. Chumas J. Tubulolobular carcinoma of the breast. Am J Surg Pathol. 1998. 22:903–904.
Article18. Mimeault M, Batra SK. New promising drug targets in cancer- and metastasis-initiating cells. Drug Discov Today. 2010. 15:354–364.
Article19. Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011. 8:97–106.
Article20. Ferrandina G, Petrillo M, Bonanno G, Scambia G. Targeting CD133 antigen in cancer. Expert Opin Ther Targets. 2009. 13:823–837.
Article21. Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008. 26:3008–3017.
Article22. Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008. 99:100–109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to: Aberrant Expression of Cancer Stem Cells Marker Prominin-1 in Low-Grade Tubulobular Breast Carcinoma: A Correlative Study between qRT-PCR, Flow-Cytometric and Immunohistochemistry Analysis
- Nuclear Expression of Mutant p53 protein in Transitinal Cell Carcinoma of the Bladder Detected by Immunohistochemistry: The Correlative Study with Proliferating Cell Nuclear Antigen Expression, Nucleolar Organizer Regions per Nucleus and Flow cytometric P
- Inhibitor of DNA-binding 4 contributes to the maintenance and expansion of cancer stem cells in 4T1 mouse mammary cancer cell line
- Comparative study on results between urine flow cytometric DNA analysis and urine cytology in transtional cell carcinoma of bladder
- The clinical significance of expression of proliferating cell nuclear antigen(PCNA) in prostatic carcinoma: the correlative study with glandular differentiation and flow cytometric DNA analysis