J Breast Cancer.
2013 Jun;16(2):178-183. 10.4048/jbc.2013.16.2.178.
Early Cardiac Function Monitoring for Detection of Subclinical Doxorubicin Cardiotoxicity in Young Adult Patients with Breast Cancer
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. younhj@catholic.ac.kr
- 2Department of Surgery, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2286398
- DOI: http://doi.org/10.4048/jbc.2013.16.2.178
Abstract
- PURPOSE
As doxorubicin cardiotoxicity is considered irreversible, early detection of cardiotoxicity and prevention of overt heart failure is essential. Although there are monitoring guidelines for cardiotoxicity, optimal timing for early detection of subclinical doxorubicin cardiotoxicity is still obscure. The purpose of this study is to determine optimal timing of cardiac monitoring and risk factors for early detection of doxorubicin cardiotoxicity in young adult patients with breast cancer.
METHODS
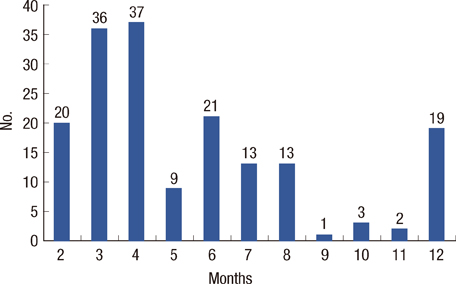
Medical records of 1,013 breast cancer patients diagnosed from January 2009 to December 2010 is being reviewed and analyzed. Properly monitored patients are defined as patients who underwent transthoracic echocardiography before and after the chemotherapy. The definition of subclinical cardiotoxicity (SC) either decreases left ventricular ejection fraction (LVEF) more than 10% or the LVEF declines under 55% from baseline without heart failure symptoms.
RESULTS
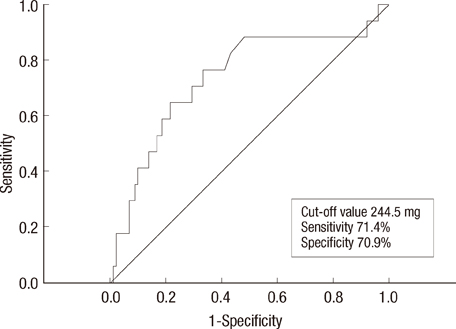
Twenty-nine out of 174 (16.7%) properly monitored young adult female patients (mean age, 52+/-10 years old) developed SC. The mean interval of cardiac evaluation of SC group was 5.5+/-3.0 months. Among the risk factors, the history of coronary artery disease, cumulative dose of doxorubicin > or =300 mg/m2 and use of trastuzumab after doxorubicin therapy were associated with development of SC. At cumulative dose of doxorubicin 244.5 mg/m2, SC can be predicted (sensitivity, 71.4%; specificity, 70.9%; area under the curve, 0.741; 95% confidence interval, 0.608-0.874; p=0.001).
CONCLUSION
In young adult patients with breast cancer, SC was common at cumulative dose of doxorubicin <300 mg/m2 and early performance of cardiac monitoring before reaching the conventional critical dose of doxorubicin might be a proper strategy for early detection of SC.
MeSH Terms
Figure
Cited by 2 articles
-
Cardiovascular Complications of Novel Anti-Cancer Immunotherapy: Old Problems from New Agents?
Woo-Baek Chung, Jong-Chan Youn, Ho-Joong Youn
Korean Circ J. 2020;50(9):743-753. doi: 10.4070/kcj.2020.0158.Chemotherapy-Induced Left Ventricular Dysfunction in Patients with Breast Cancer
Hyun Ju Yoon, Kye Hun Kim, Jong Yoon Kim, Hyuk Jin Park, Jae Yeong Cho, Young Joon Hong, Hyung Wook Park, Ju Han Kim, Youngkeun Ahn, Myung Ho Jeong, Jeong Gwan Cho, Jong Chun Park
J Breast Cancer. 2016;19(4):402-409. doi: 10.4048/jbc.2016.19.4.402.
Reference
-
1. Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010; 53:105–113.
Article2. Youn HJ, Kim HS, Jeon MH, Lee JH, Seo YJ, Lee YJ, et al. Induction of caspase-independent apoptosis in H9c2 cardiomyocytes by adriamycin treatment. Mol Cell Biochem. 2005; 270:13–19.
Article3. Chung WB, Youn HJ, Choi YS, Park CS, Oh YS, Chung WS, et al. The expression of cardiac ankyrin repeat protein in an animal model of adriamycin-induced cardiomyopathy. Korean Circ J. 2008; 38:455–461.
Article4. Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991; 266:1672–1677.
Article5. Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br J Cancer. 2002; 86:1697–1700.
Article6. Belham M, Kruger A, Mepham S, Faganello G, Pritchard C. Monitoring left ventricular function in adults receiving anthracycline-containing chemotherapy. Eur J Heart Fail. 2007; 9:409–414.
Article7. Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies are clinicians responding optimally? J Am Coll Cardiol. 2010; 56:1644–1650.
Article8. Steinherz LJ, Graham T, Hurwitz R, Sondheimer HM, Schwartz RG, Shaffer EM, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics. 1992; 89(5 Pt 1):942–949.
Article9. Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009; 27:127–145.
Article10. van Dalen EC, van den Brug M, Caron HN, Kremer LC. Anthracycline-induced cardiotoxicity: comparison of recommendations for monitoring cardiac function during therapy in paediatric oncology trials. Eur J Cancer. 2006; 42:3199–3205.
Article11. Jannazzo A, Hoffman J, Lutz M. Monitoring of anthracycline-induced cardiotoxicity. Ann Pharmacother. 2008; 42:99–104.
Article12. Nagy AC, Cserép Z, Tolnay E, Nagykálnai T, Forster T. Early diagnosis of chemotherapy-induced cardiomyopathy: a prospective tissue Doppler imaging study. Pathol Oncol Res. 2008; 14:69–77.
Article13. Jassal DS, Han SY, Hans C, Sharma A, Fang T, Ahmadie R, et al. Utility of tissue Doppler and strain rate imaging in the early detection of trastuzumab and anthracycline mediated cardiomyopathy. J Am Soc Echocardiogr. 2009; 22:418–424.
Article14. Di Lisi D, Bonura F, Macaione F, Peritore A, Meschisi M, Cuttitta F, et al. Chemotherapy-induced cardiotoxicity: role of the tissue Doppler in the early diagnosis of left ventricular dysfunction. Anticancer Drugs. 2011; 22:468–472.15. Ho E, Brown A, Barrett P, Morgan RB, King G, Kennedy MJ, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart. 2010; 96:701–707.
Article16. Stoodley PW, Richards DA, Hui R, Boyd A, Harnett PR, Meikle SR, et al. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr. 2011; 12:945–952.
Article17. Lightfoot JC, D'Agostino RB Jr, Hamilton CA, Jordan J, Torti FM, Kock ND, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010; 3:550–558.
Article18. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011; 107:1375–1380.
Article19. Bovelli D, Plataniotis G, Roila F. ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010; 21:Suppl 5. v277–v282.
Article20. Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008; 14:14–24.
Article21. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010; 55:213–220.22. De Keyzer E, Kerkhove D, Van Camp G, De Sutter J, Achtergael W, Keymeulen B, et al. Screening for silent myocardial ischaemia in patients with type 2 diabetes mellitus: a quest to improve selection of the target screening population. Acta Cardiol. 2011; 66:715–720.
Article23. Perez EA, Suman VJ, Davidson NE, Kaufman PA, Martino S, Dakhil SR, et al. Effect of doxorubicin plus cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the North Central Cancer Treatment Group N9831 Intergroup Adjuvant Trial. J Clin Oncol. 2004; 22:3700–3704.
Article24. Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf. 2008; 31:459–467.
Article25. Butany J, Ahn E, Luk A. Drug-related cardiac pathology. J Clin Pathol. 2009; 62:1074–1084.
Article26. Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, et al. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009; 100:684–692.
Article27. Subar M, Lin W, Chen W, Pittman DG. Lack of uniformity in cardiac assessment during trastuzumab therapy. Breast J. 2011; 17:383–390.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Left Ventricular Diastolic Function in Patients Receiving Doxorubicin
- Loss of endogenous estrogen increases cardiac toxicity by doxorubicin
- Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity
- Breast Cancer and Therapy-Related Cardiovascular Toxicity
- Cardiovascular Complications of Novel Anti-Cancer Immunotherapy: Old Problems from New Agents?