Korean Circ J.
2020 Sep;50(9):743-753. 10.4070/kcj.2020.0158.
Cardiovascular Complications of Novel Anti-Cancer Immunotherapy: Old Problems from New Agents?
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2505730
- DOI: http://doi.org/10.4070/kcj.2020.0158
Abstract
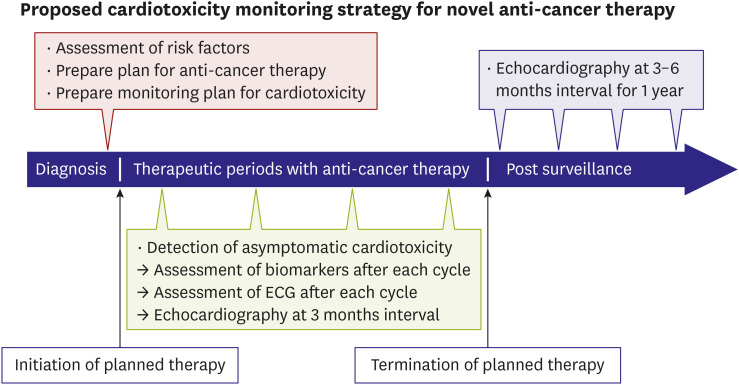
- Many novel anti-cancer therapies have dramatically improved outcomes of various cancer patients. However, it also poses a risk for cardiovascular complications as well. For the novel anti-cancer agent with which physicians does not have enough clinical experiences to determine the characteristics of cardiovascular complications, it is important to assess risk factors for cardiotoxicity before starting anti-cancer therapy. High-risk patient should be consulted to cardiologist before initiating anti-cancer therapy and pre-emptive cardiac function monitoring plan might be prepared in advance. The biomarkers, electrocardiography and echocardiography are useful tools for the detection of subclinical cardiotoxicity during anti-cancer therapy. This review article tried to suggest the cardiac function monitoring strategies for newly encountered potential cardiotoxic anti-cancer agents and to summarize the cardiovascular complications of novel anti-cancer immunotherapies including immune checkpoint inhibitor (ICI) and chimeric antigen receptor (CAR) T-cell therapy. ICIs can cause fatal myocarditis, which usually occurs early after initiation, and prompt treatment with high-dose corticosteroid is necessary. CAR T-cell therapy can cause cytokine release syndrome, which may result in circulatory collapse. Supportive treatment as well as tocilizumab, an anti-interleukin-6 receptor antibody are cornerstones of treatment.
Keyword
Figure
Reference
-
1. Pareek N, Cevallos J, Moliner P, et al. Activity and outcomes of a cardio-oncology service in the United Kingdom-a five-year experience. Eur J Heart Fail. 2018; 20:1721–1731. PMID: 30191649.
Article2. Masoudkabir F, Sarrafzadegan N, Gotay C, et al. Cardiovascular disease and cancer: evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis. 2017; 263:343–351. PMID: 28624099.
Article3. Meijers WC, de Boer RA. Common risk factors for heart failure and cancer. Cardiovasc Res. 2019; 115:844–853. PMID: 30715247.
Article4. Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014; 15:1063–1093. PMID: 25239940.
Article5. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016; 37:2768–2801. PMID: 27567406.6. Kim H, Chung WB, Cho KI, et al. Diagnosis, treatment, and prevention of cardiovascular toxicity related to anti-cancer treatment in clinical practice: an opinion paper from the working group on cardio-oncology of the Korean Society of Echocardiography. J Cardiovasc Ultrasound. 2018; 26:1–25. PMID: 29629020.
Article7. Youn JC, Han S, Ryu KH. Temporal trends of hospitalized patients with heart failure in Korea. Korean Circ J. 2017; 47:16–24. PMID: 28154584.
Article8. Choi HM, Park MS, Youn JC. Update on heart failure management and future directions. Korean J Intern Med. 2019; 34:11–43. PMID: 30612416.
Article9. Kim DY, Youn JC, Park MS, et al. Cardiovascular outcome of breast cancer patients with concomitant radiotherapy and chemotherapy: a 10-year multicenter cohort study. J Cardiol. 2019; 74:175–181. PMID: 30827728.
Article10. Kim KJ, Cho HJ, Kim MS, et al. Focused update of 2016 Korean Society of Heart failure guidelines for the management of chronic heart failure. Int J Heart Fail. 2019; 1:4–24.
Article11. Lee JH, Kim MS, Yoo BS, et al. KSHF guidelines for the management of acute heart failure: part II. Treatment of acute heart failure. Korean Circ J. 2019; 49:22–45. PMID: 30637994.
Article12. Stewart Coats AJ. Common co-morbidities in heart failure – diabetes, functional mitral regurgitation and sleep apnoea. Int J Heart Fail. 2019; 1:25–41.
Article13. Lee HY, Oh BH. Paradigm shifts of heart failure therapy: do we need another paradigm? Int J Heart Fail. 2020.
Article14. Uhm JS, Chung WB, Yoon JS, Oh YS, Youn HJ. Effects of adriamycin and candesartan on the collagen and elastin of the aorta in rats. Clin Hypertens. 2014; 20:8. PMID: 26909195.
Article15. Chung WB, Youn HJ. Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity. Korean J Intern Med. 2016; 31:625–633. PMID: 27378126.
Article16. Chung WB, Yi JE, Jin JY, et al. Early cardiac function monitoring for detection of subclinical doxorubicin cardiotoxicity in young adult patients with breast cancer. J Breast Cancer. 2013; 16:178–183. PMID: 23843850.
Article17. Gripp EA, Oliveira GE, Feijó LA, Garcia MI, Xavier SS, Sousa AS. Global longitudinal strain accuracy for cardiotoxicity prediction in a cohort of breast cancer patients during anthracycline and/or trastuzumab treatment. Arq Bras Cardiol. 2018; 110:140–150. PMID: 29561992.18. Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017; 35:893–911. PMID: 27918725.
Article19. Virizuela JA, García AM, de Las Peñas R, et al. SEOM clinical guidelines on cardiovascular toxicity (2018). Clin Transl Oncol. 2019; 21:94–105. PMID: 30627982.
Article20. Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020; 31:171–190. PMID: 31959335.
Article21. Zardavas D, Suter TM, Van Veldhuisen DJ, et al. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2-positive breast cancer receiving trastuzumab: a Herceptin Adjuvant study cardiac marker substudy. J Clin Oncol. 2017; 35:878–884. PMID: 28199174.
Article22. Michel L, Mincu RI, Mahabadi AA, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020; 22:350–361. PMID: 31721381.
Article23. Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of anthracyclines. Front Cardiovasc Med. 2020; 7:26. PMID: 32258060.
Article24. Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J Thorac Dis. 2018; 10:S4282–95. PMID: 30701097.
Article25. Kittiwarawut A, Vorasettakarnkij Y, Tanasanvimon S, Manasnayakorn S, Sriuranpong V. Serum NT-proBNP in the early detection of doxorubicin-induced cardiac dysfunction. Asia Pac J Clin Oncol. 2013; 9:155–161. PMID: 22897825.
Article26. Negishi T, Miyazaki S, Negishi K. Echocardiography and cardio-oncology. Heart Lung Circ. 2019; 28:1331–1338. PMID: 31230869.
Article27. Ganatra S, Parikh R, Neilan TG. Cardiotoxicity of immune therapy. Cardiol Clin. 2019; 37:385–397. PMID: 31587780.
Article28. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018; 378:158–168. PMID: 29320654.
Article29. Ganatra S, Carver JR, Hayek SS, et al. Chimeric antigen receptor T-cell therapy for cancer and heart: JACC council perspectives. J Am Coll Cardiol. 2019; 74:3153–3163. PMID: 31856973.30. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016; 375:1749–1755. PMID: 27806233.31. Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018; 71:1755–1764. PMID: 29567210.
Article32. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018; 391:933.
Article33. Lyon AR, Yousaf N, Battisti NM, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018; 19:e447–58. PMID: 30191849.
Article34. Bonaca MP, Olenchock BA, Salem JE, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019; 140:80–91. PMID: 31390169.35. Escudier M, Cautela J, Malissen N, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017; 136:2085–2087. PMID: 29158217.
Article36. Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018; 19:1579–1589. PMID: 30442497.
Article37. Obstfeld AE, Frey NV, Mansfield K, et al. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights. Blood. 2017; 130:2569–2572. PMID: 29074500.
Article38. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017; 377:2531–2544. PMID: 29226797.39. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018; 378:439–448. PMID: 29385370.40. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019; 380:45–56. PMID: 30501490.41. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020; 9:e013757. PMID: 31960755.
Article42. Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res. 2007; 101:248–257. PMID: 17569889.43. Okazaki T, Tanaka Y, Nishio R, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003; 9:1477–1483. PMID: 14595408.
Article44. Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001; 291:319–322. PMID: 11209085.
Article45. Lichtman AH. The heart of the matter: protection of the myocardium from T cells. J Autoimmun. 2013; 45:90–96. PMID: 23810579.
Article46. Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012; 188:4876–4884. PMID: 22491251.
Article47. Pathan N, Hemingway CA, Alizadeh AA, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004; 363:203–209. PMID: 14738793.
Article48. Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013; 122:863–871. PMID: 23770775.
Article49. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018; 36:1714–1768. PMID: 29442540.50. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009; 53:1475–1487. PMID: 19389557.
Article51. Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018; 72:3158–3176. PMID: 30545455.52. Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004; 109:1250–1258. PMID: 14993139.
Article53. Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005; 45:1815–1822. PMID: 15936612.
Article54. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019; 25:625–638. PMID: 30592986.
Article55. Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018; 23:943–947. PMID: 29622697.
Article56. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014; 6:224ra25.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cancer Immunotherapy: The Dawn of the Renaissance after the Medieval Dark Ages
- Current Development Status of Cytokines for Cancer Immunotherapy
- Immunotherapy for Advanced/Metastatic Esophageal Squamous Cell Carcinoma
- The Effectiveness of Long-Term Immunotherapy with Every 3-Month Injection after 3-Year Immunotherapy in the Treatment of Perennial Allergic Rhinitis Using Quality of Life Questionnaires
- Cutaneous Adverse Reactions of New Anti-cancer Agents