J Bacteriol Virol.
2013 Sep;43(3):177-185. 10.4167/jbv.2013.43.3.177.
Development and Application of Cell-penetrating Peptides
- Affiliations
-
- 1Division of Influenza Virus, Center for Infectious Diseases, Korea National Institute of Health, Korea Centers for Disease Control and Prevention, Chungcheongbuk-do, Korea. pobee@nih.go.kr
- KMID: 2286271
- DOI: http://doi.org/10.4167/jbv.2013.43.3.177
Abstract
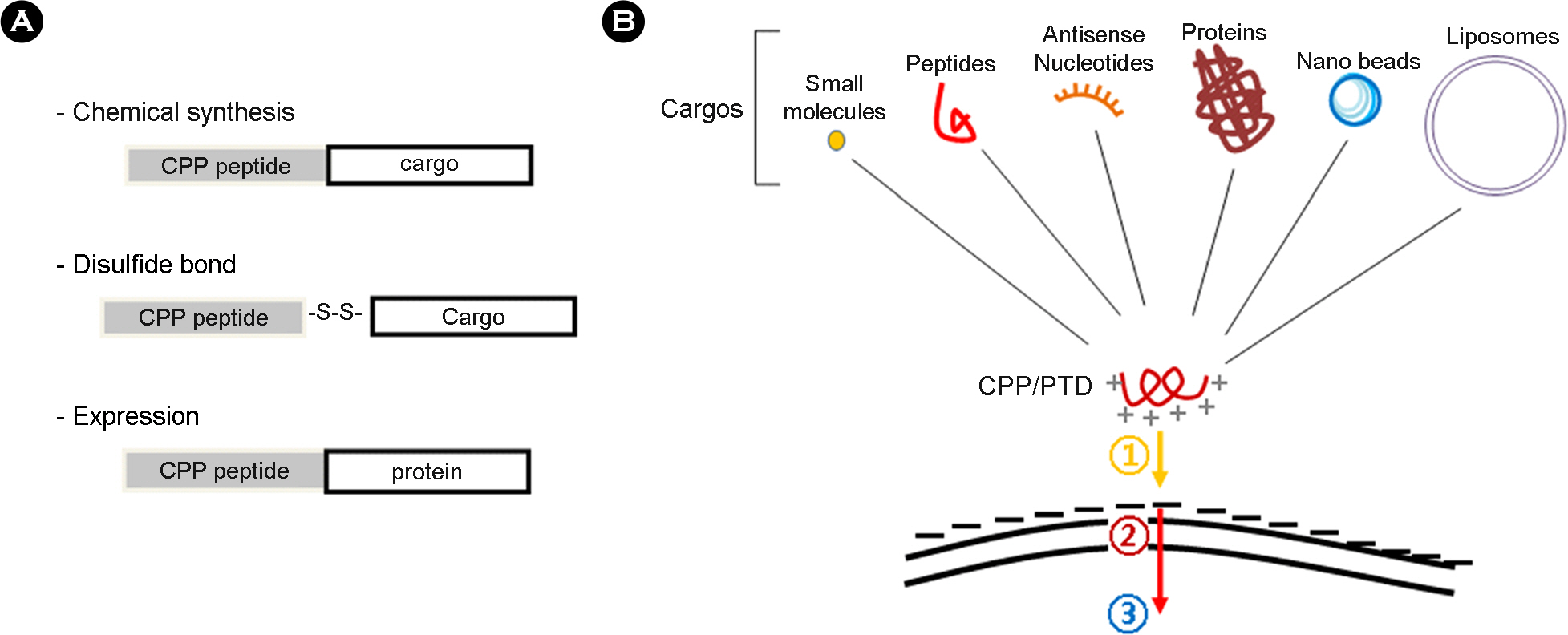
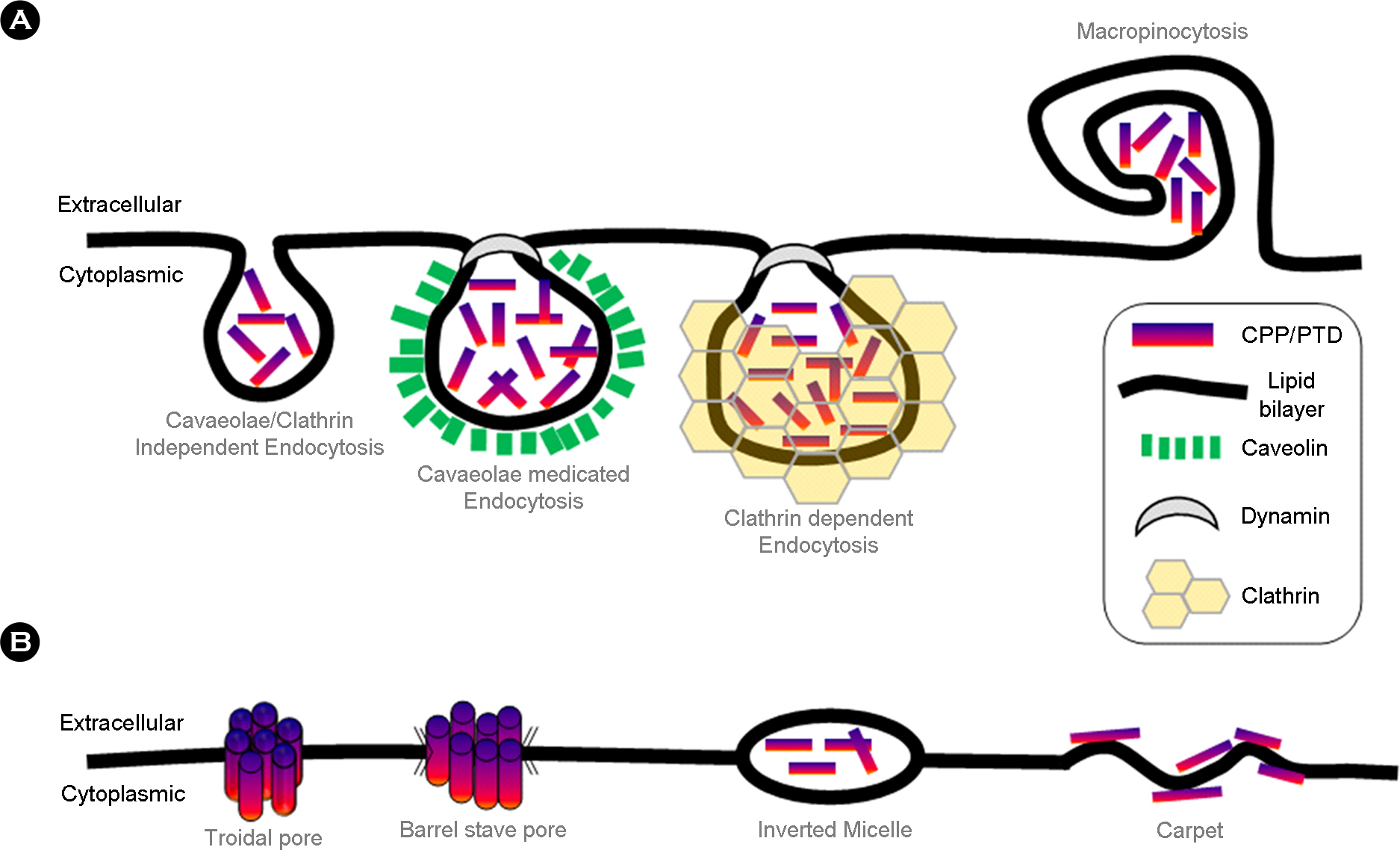
- Intracellular transduction of hydrophilic macromolecules has been problematic owing to the biochemical restriction imposed by lipid bilayer of the cytoplasmic membrane. Several technologies have been developed to improve the intracellular delivery of the large molecules for therapeutic purpose, including cell penetrating peptide. Cell penetrating peptides or cell permeable peptides (CPPs) were initially discovered based on the potency of certain full-length proteins or proteins to translocate across the plasma membrane. Currently, CPPs are broadly applied for intracellular delivery of biologically functional molecules in vivo and vitro, varying from small molecules, peptides, proteins, liposomes and nucleic acids. With introducing the history and characteristics of CPPs, this review will focus on the intracellular transduction mechanism and application of CPPs.
MeSH Terms
Figure
Cited by 1 articles
-
Antimicrobial Proteins in Intestine and Inflammatory Bowel Diseases
Jung Mogg Kim
Intest Res. 2014;12(1):20-33. doi: 10.5217/ir.2014.12.1.20.
Reference
-
1). Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004; 6:189–96.
Article2). Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. 2002; 13:52–6.
Article3). Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009; 157:195–206.
Article4). Frankel AD, Pabo CO. Cellular uptake of the Tat protein from human immunodeficiency virus. Cell. 1988; 55:1189–93.
Article5). Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001; 276:5836–40.6). Berlose JP, Convert O, Derossi D, Brunissen A, Chassaing G. Conformational and associative behaviors of the third helix of Antennapedia homeodomain in membrane-mimetic environments. Eur J Biochem. 1996; 242:372–86.7). Drin G, Déméné H, Temsamani J, Brasseur R. Translocation of the pAntp peptide and its amphipathic analogue AP-2AL. Biochemistry. 2001; 40:1824–34.
Article8). Scheller A, Wiesner B, Melzig M, Bienert M, Oehlke J. Evidence for an amphipathicity independent cellular uptake of amphipathic cell-penetrating peptides. Eur J Biochem. 2000; 267:6043–50.
Article9). Trabulo S, Cardoso AL, Mano M, de Lima MC. Cell-Penetrating Peptides-Mechanisms of Cellular Uptake and Generation of Delivery Systems. Pharmaceuticals. 2010; 3:961–93.
Article10). Vivès E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997; 272:16010–7.11). Schmidt N, Mishra A, Lai GH, Wong GC. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010; 584:1806–13.
Article12). Pietersz GA, Li W, Apostolopoulos V. A 16-mer peptide (RQIKIWFQNRRMKWKK) from antennapedia preferentially targets the Class I pathway. Vaccine. 2001; 19:1397–405.
Article13). Vasconcelos L, Pärn K, Langel U. Therapeutic potential of cell-penetrating peptides. Ther Deliv. 2013; 4:573–91.
Article14). Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006; 12:574–9.
Article15). Gehring WJ. Homeo boxes in the study of development. Science. 1987; 236:1245–52.
Article16). Brugidou J. Legrand C, Méry J, Rabié A. The retro-inverso form of a homeobox-derived short peptide is rapidly internalised by cultured neurons: a new basis for an efficient intracellular delivery system. Biochem Biophys Res Commun. 1995; 214:685–93.17). Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K, Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J Biol Chem. 2002; 277:2437–43.
Article18). Futaki S. Membrane-permeable arginine-rich peptides and the translocation mechanisms. Adv Drug Deliv Rev. 2005; 57:547–58.
Article19). Noguchi H, Matsushita M, Okitsu T, Moriwaki A, Tomizawa K, Kang S, et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med. 2004; 10:305–9.
Article20). Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005; 11:892–8.
Article21). Järver P, Langel U. The use of cell-penetrating peptides as a tool for gene regulation. Drug Discov Today. 2004; 9:395–402.22). Laufer SD, Restle T. Peptide-mediated cellular delivery of oligonucleotide-based therapeutics in vitro: quantitative evaluation of overall efficacy employing easy to handle reporter systems. Curr Pharm Des. 2008; 14:3637–55.23). Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer. 2005; 5:468–79.
Article24). Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008; 36:4158–71.
Article25). Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003; 270:1628–44.
Article26). Lebleu B, Moulton HM, Abes R, Ivanova GD, Abes S, Stein DA, et al. Cell penetrating peptide conjugates of steric block oligonucleotides. Adv Drug Deliv Rev. 2008; 60:517–29.
Article27). Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008; 90:604–10.
Article28). Temsamani J, Vidal P. The use of cell-penetrating peptides for drug delivery. Drug Discov Today. 2004; 9:1012–9.
Article29). Hannon GJ. RNA interference. Nature. 2002; 418:244–51.
Article30). Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov. 2004; 3:318–29.
Article31). de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007; 6:443–53.
Article32). Muratovska A, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004; 558:63–8.
Article33). Chiu YL, Ali A, Chu CY, Cao H, Rana TM. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Biol. 2004; 11:1165–75.
Article34). Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, et al. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004; 24:10040–6.
Article35). Zeineddine D, Papadimou E, Chebli K, Gineste M, Liu J, Grey C, et al. Oct-3/4 dose dependently regulates specification of embryonic stem cells toward a cardiac lineage and early heart development. Dev Cell. 2006; 11:535–46.
Article36). Crombez L, Morris MC, Dufort S, Aldrian-Herrada G, Nguyen Q, Mc Master G, et al. Targeting cyclin B1 through peptide-based delivery of siRNA prevents tumour growth. Nucleic Acids Res. 2009; 37:4559–69.
Article37). Eguchi A, Meade BR, Chang YC, Fredrickson CT, Willert K, Puri N, et al. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat Biotechnol. 2009; 27:567–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of Cell-Penetrating Peptides in Dendritic Cell-Based Vaccination
- Priming of Autoreactive CD8+ T Cells Is Inhibited by Immunogenic Peptides Which Are Competitive for Major Histocompatibility Complex Class I Binding
- Recent Trends in Cyclic Peptides as Therapeutic Agents and Biochemical Tools
- Endothelial Cell Changes after Penetrating Keratoplasty
- Measurements of Dynamic Contour Tonometry After Penetrating Keratoplasty and EpiLASIK