Korean J Physiol Pharmacol.
2015 Jul;19(4):349-355. 10.4196/kjpp.2015.19.4.349.
Antinociceptive Effects of Transcytosed Botulinum Neurotoxin Type A on Trigeminal Nociception in Rats
- Affiliations
-
- 1Department of Oral Physiology, School of Dentistry, Kyungpook National University, Daegu 700-412, Korea. dkahn@knu.ac.kr
- 2Department of Oral Anatomy, School of Dentistry, Kyungpook National University, Daegu 700-412, Korea.
- 3Department of Orofacial Pain and Oral Medicine, School of Dentistry, Yonsei University, Seoul 110-749, Korea.
- KMID: 2285578
- DOI: http://doi.org/10.4196/kjpp.2015.19.4.349
Abstract
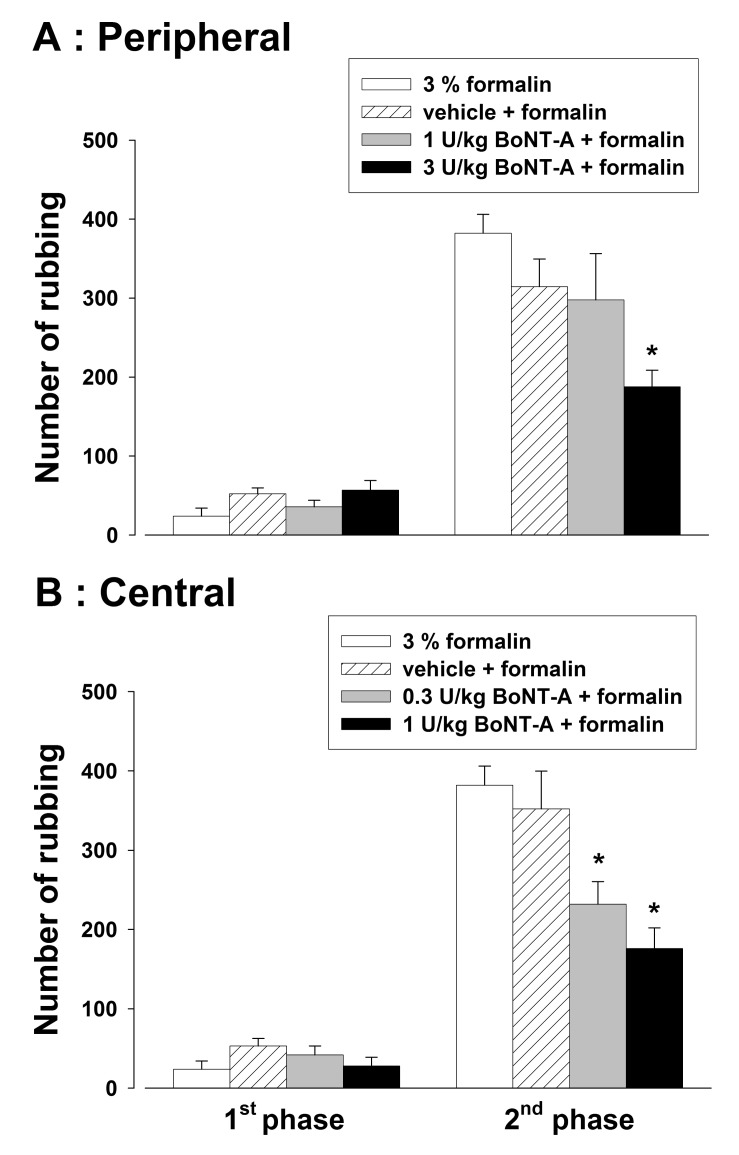
- We examined the effects of peripherally or centrally administered botulinum neurotoxin type A (BoNT-A) on orofacial inflammatory pain to evaluate the antinociceptive effect of BoNT-A and its underlying mechanisms. The experiments were carried out on male Sprague-Dawley rats. Subcutaneous (3 U/kg) or intracisternal (0.3 or 1 U/kg) administration of BoNT-A significantly inhibited the formalin-induced nociceptive response in the second phase. Both subcutaneous (1 or 3 U/kg) and intracisternal (0.3 or 1 U/kg) injection of BoNT-A increased the latency of head withdrawal response in the complete Freund's adjuvant (CFA)-treated rats. Intracisternal administration of N-methyl-D-aspartate (NMDA) evoked nociceptive behavior via the activation of trigeminal neurons, which was attenuated by the subcutaneous or intracisternal injection of BoNT-A. Intracisternal injection of NMDA up-regulated c-Fos expression in the trigeminal neurons of the medullary dorsal horn. Subcutaneous (3 U/kg) or intracisternal (1 U/kg) administration of BoNT-A significantly reduced the number of c-Fos immunoreactive neurons in the NMDA-treated rats. These results suggest that the central antinociceptive effects the peripherally or centrally administered BoNT-A are mediated by transcytosed BoNT-A or direct inhibition of trigeminal neurons. Our data suggest that central targets of BoNT-A might provide a new therapeutic tool for the treatment of orofacial chronic pain conditions.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Botulinum toxin type A enhances the inhibitory spontaneous postsynaptic currents on the substantia gelatinosa neurons of the subnucleus caudalis in immature mice
Seon-Hui Jang, Soo-Joung Park, Chang-Jin Lee, Dong-Kuk Ahn, Seong-Kyu Han
Korean J Physiol Pharmacol. 2018;22(5):539-546. doi: 10.4196/kjpp.2018.22.5.539.Participation of central GABAA receptors in the trigeminal processing of mechanical allodynia in rats
Min Ji Kim, Young Hong Park, Kui Ye Yang, Jin Sook Ju, Yong Chul Bae, Seong Kyu Han, Dong Kuk Ahn
Korean J Physiol Pharmacol. 2017;21(1):65-74. doi: 10.4196/kjpp.2017.21.1.65.
Reference
-
1. de Paiva A, Poulain B, Lawrence GW, Shone CC, Tauc L, Dolly JO. A role for the interchain disulfide or its participating thiols in the internalization of botulinum neurotoxin A revealed by a toxin derivative that binds to ecto-acceptors and inhibits transmitter release intracellularly. J Biol Chem. 1993; 268:20838–20844. PMID: 8104936.
Article2. Rizo J, Südhof TC. Mechanics of membrane fusion. Nat Struct Biol. 1998; 5:839–842. PMID: 9783736.
Article3. Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000; 80:717–766. PMID: 10747206.
Article4. Rollnik JD, Tanneberger O, Schubert M, Schneider U, Dengler R. Treatment of tension-type headache with botulinum toxin type A: a double-blind, placebo-controlled study. Headache. 2000; 40:300–305. PMID: 10759934.
Article5. Foster L, Clapp L, Erickson M, Jabbari B. Botulinum toxin A and chronic low back pain: a randomized, double-blind study. Neurology. 2001; 56:1290–1293. PMID: 11376175.
Article6. Sheean G. Botulinum toxin for the treatment of musculoskeletal pain and spasm. Curr Pain Headache Rep. 2002; 6:460–469. PMID: 12413405.
Article7. Silberstein S, Mathew N, Saper J, Jenkins S. For the BOTOX Migraine Clinical Research Group. Botulinum toxin type A as a migraine preventive treatment. Headache. 2000; 40:445–450. PMID: 10849039.
Article8. Smuts JA, Schultz D, Barnard A. Mechanism of action of botulinum toxin type A in migraine prevention: a pilot study. Headache. 2004; 44:801–805. PMID: 15330827.
Article9. Göbel H, Heinze A, Heinze-Kuhn K, Austermann K. Botulinum toxin A in the treatment of headache syndromes and pericranial pain syndromes. Pain. 2001; 91:195–199. PMID: 11275374.
Article10. Bach-Rojecky L, Lacković Z. Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain. Croat Med J. 2005; 46:201–208. PMID: 15849840.11. Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004; 107:125–133. PMID: 14715398.
Article12. Luvisetto S, Marinelli S, Lucchetti F, Marchi F, Cobianchi S, Rossetto O, Montecucco C, Pavone F. Botulinum neurotoxins and formalin-induced pain: central vs. peripheral effects in mice. Brain Res. 2006; 1082:124–131. PMID: 16524562.
Article13. Lee WH, Shin TJ, Kim HJ, Lee JK, Suh HW, Lee SC, Seo K. Intrathecal administration of botulinum neurotoxin type A attenuates formalin-induced nociceptive responses in mice. Anesth Analg. 2011; 112:228–235. PMID: 21081780.
Article14. Bach-Rojecky L, Lacković Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009; 94:234–238. PMID: 19732788.
Article15. Kitamura Y, Matsuka Y, Spigelman I, Ishihara Y, Yamamoto Y, Sonoyama W, Kamioka H, Yamashiro T, Kuboki T, Oguma K. Botulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience. 2009; 159:1422–1429. PMID: 19409226.
Article16. Gazerani P, Staahl C, Drewes AM, Arendt-Nielsen L. The effects of Botulinum Toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain. 2006; 122:315–325. PMID: 16677761.
Article17. Borodic GE, Acquadro MA. The use of botulinum toxin for the treatment of chronic facial pain. J Pain. 2002; 3:21–27. PMID: 14622850.
Article18. Ahn DK, Chae JM, Choi HS, Kyung HM, Kwon OW, Park HS, Youn DH, Bae YC. Central cyclooxygenase inhibitors reduced IL-1beta-induced hyperalgesia in temporomandibular joint of freely moving rats. Pain. 2005; 117:204–213. PMID: 16098663.19. Wang XM, Zhang ZJ, Bains R, Mokha SS. Effect of antisense knock-down of alpha(2a)- and alpha(2c)-adrenoceptors on the antinociceptive action of clonidine on trigeminal nociception in the rat. Pain. 2002; 98:27–35. PMID: 12098614.20. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–1036. PMID: 14677603.
Article21. Choi HS, Ju JS, Lee HJ, Jung CY, Kim BC, Park JS, Ahn DK. Effects of TNF-alpha injected intracisternally on the nociceptive jaw-opening reflex and orofacial formalin test in freely moving rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003; 27:613–618. PMID: 12787847.22. Chang KH, Bai SJ, Lee H, Lee BH. Effects of acupuncture stimulation at different acupoints on formalin-induced pain in rats. Korean J Physiol Pharmacol. 2014; 18:121–127. PMID: 24757373.
Article23. Clavelou P, Pajot J, Dallel R, Raboisson P. Application of the formalin test to the study of orofacial pain in the rat. Neurosci Lett. 1989; 103:349–353. PMID: 2812522.
Article24. Yang GY, Woo YW, Park MK, Bae YC, Ahn DK, Bonfa E. Intracisternal administration of NR2 antagonists attenuates facial formalin-induced nociceptive behavior in rats. J Orofac Pain. 2010; 24:203–211. PMID: 20401359.25. Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992; 51:5–17. PMID: 1454405.
Article26. Yang KY, Mun JH, Park KD, Kim MJ, Ju JS, Kim ST, Bae YC, Ahn DK. Blockade of spinal glutamate recycling produces paradoxical antinociception in rats with orofacial inflammatory pain. Prog Neuropsychopharmacol Biol Psychiatry. 2015; 57:100–109. PMID: 25445477.
Article27. Ahn DK, Lee SY, Han SR, Ju JS, Yang GY, Lee MK, Youn DH, Bae YC. Intratrigeminal ganglionic injection of LPA causes neuropathic pain-like behavior and demyelination in rats. Pain. 2009; 146:114–120. PMID: 19665300.
Article28. Kim MJ, Lee SY, Yang KY, Nam SH, Kim HJ, Kim YJ, Bae YC, Ahn DK. Differential regulation of peripheral IL-1β-induced mechanical allodynia and thermal hyperalgesia in rats. Pain. 2014; 155:723–732. PMID: 24406203.
Article29. Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, Kim JS, Oh SB. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain. 2009; 144:84–94. PMID: 19376653.
Article30. Kim HD, Lee HJ, Choi HS, Ju JS, Jung CY, Bae YC, Ahn DK. Interleukin-1 beta injected intracisternally inhibited NMDA-evoked behavioral response in the orofacial area of freely moving rats. Neurosci Lett. 2004; 360:37–40. PMID: 15082173.31. Lee HJ, Choi HS, Jung CY, Ju JS, Kim SK, Bae YC, Ahn DK. Intracisternal NMDA produces analgesia in the orofacial formalin test of freely moving rats. Prog Neuropsychopharmacol Biol Psychiatry. 2004; 28:497–503. PMID: 15093957.
Article32. Wilcox GL. Pharmacological studies of grooming and scratching behavior elicited by spinal substance P and excitatory amino acids. Ann N Y Acad Sci. 1988; 525:228–236. PMID: 2455461.
Article33. Zhang KM, Wang XM, Peterson AM, Chen WY, Mokha SS. alpha2-adrenoceptors modulate NMDA-evoked responses of neurons in superficial and deeper dorsal horn of the medulla. J Neurophysiol. 1998; 80:2210–2214. PMID: 9772273.34. Dolly JO, Aoki KR. The structure and mode of action of different botulinum toxins. Eur J Neurol. 2006; 13(Suppl 4):1–9. PMID: 17112344.
Article35. Simpson LL. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980; 212:16–21. PMID: 6243359.36. Hua Y, Scheller RH. Three SNARE complexes cooperate to mediate membrane fusion. Proc Natl Acad Sci U S A. 2001; 98:8065–8070. PMID: 11427709.
Article37. Ahnert-Hilger G, Bigalke H. Molecular aspects of tetanus and botulinum neurotoxin poisoning. Prog Neurobiol. 1995; 46:83–96. PMID: 7568911.
Article38. Dutton JJ, Buckley EG. Botulinum toxin in the management of blepharospasm. Arch Neurol. 1986; 43:380–382. PMID: 3954621.
Article39. Matak I, Stracenski I, Lacković Z. Comparison of analgesic effects of single versus repeated injection of botulinum toxin in orofacial formalin test in rats. J Neural Transm. 2013; 120:141–144. PMID: 22706994.
Article40. Yoo KY, Lee HS, Cho YK, Lim YS, Kim YS, Koo JH, Yoon SJ, Lee JH, Jang KH, Song SH. Anti-inflammatory effects of botulinum toxin type a in a complete Freund's adjuvant-induced arthritic knee joint of hind leg on rat model. Neurotox Res. 2014; 26:32–39. PMID: 24338136.
Article41. Marinelli S, Vacca V, Ricordy R, Uggenti C, Tata AM, Luvisetto S, Pavone F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS One. 2012; 7:e47977. PMID: 23110146.
Article42. Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon. 2000; 38:245–258. PMID: 10665805.
Article43. Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A). J Neurosci. 2011; 31:15650–15659. PMID: 22049408.
Article44. Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008; 28:3689–3696. PMID: 18385327.
Article45. Marinelli S, Luvisetto S, Cobianchi S, Makuch W, Obara I, Mezzaroma E, Caruso M, Straface E, Przewlocka B, Pavone F. Botulinum neurotoxin type A counteracts neuropathic pain and facilitates functional recovery after peripheral nerve injury in animal models. Neuroscience. 2010; 171:316–328. PMID: 20826198.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Mechanism of the Action of Clostridium botulinum Type B Neurotoxin
- The Antinociceptive and Antiallodynic Effects by Brimonidine, a Selective alpha2 Adrenergic Agonist
- A clinical study on the use of botulinum toxin type a in maxillofacial area
- Antinociceptive Effect of Intraperitoneally Administered 5,5-dimethyl-1-pyrroline N-oxide on Formalin Induced Nociception in Rats
- Treatment of Winkles and Hyperhidrosis with Botulinum Toxin Type A