Korean J Physiol Pharmacol.
2015 Mar;19(2):125-130. 10.4196/kjpp.2015.19.2.125.
Effect of the Combination of CI-988 and Morphine on Neuropathic Pain after Spinal Cord Injury in Rats
- Affiliations
-
- 1Department of Physical Therapy, Korea University College of Health Science, Seoul 136-703, Korea. junokim@korea.ac.kr
- 2Rehabilitation Science Program, Korea University College of Health Science, Seoul 136-703, Korea.
- 3Department of Physiology, Korea University College of Medicine, Seoul 136-705, Korea. ywyoon@korea.ac.kr
- KMID: 2285564
- DOI: http://doi.org/10.4196/kjpp.2015.19.2.125
Abstract
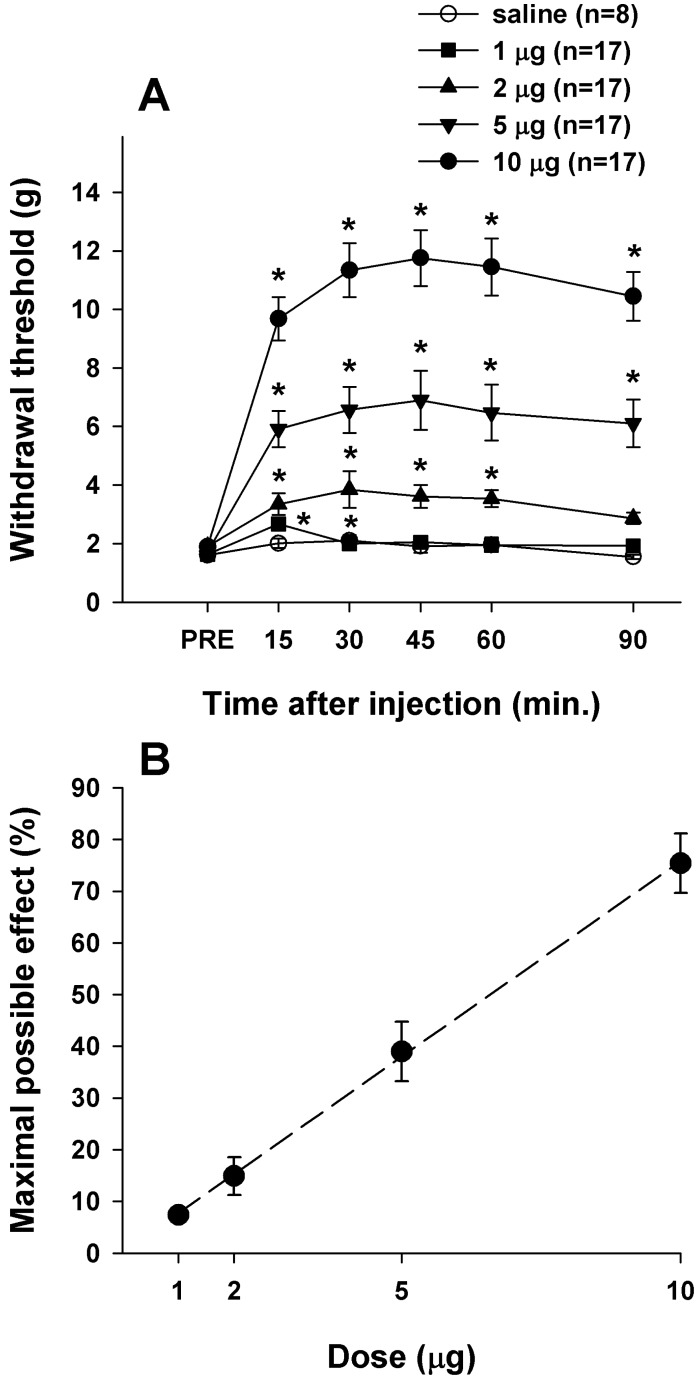
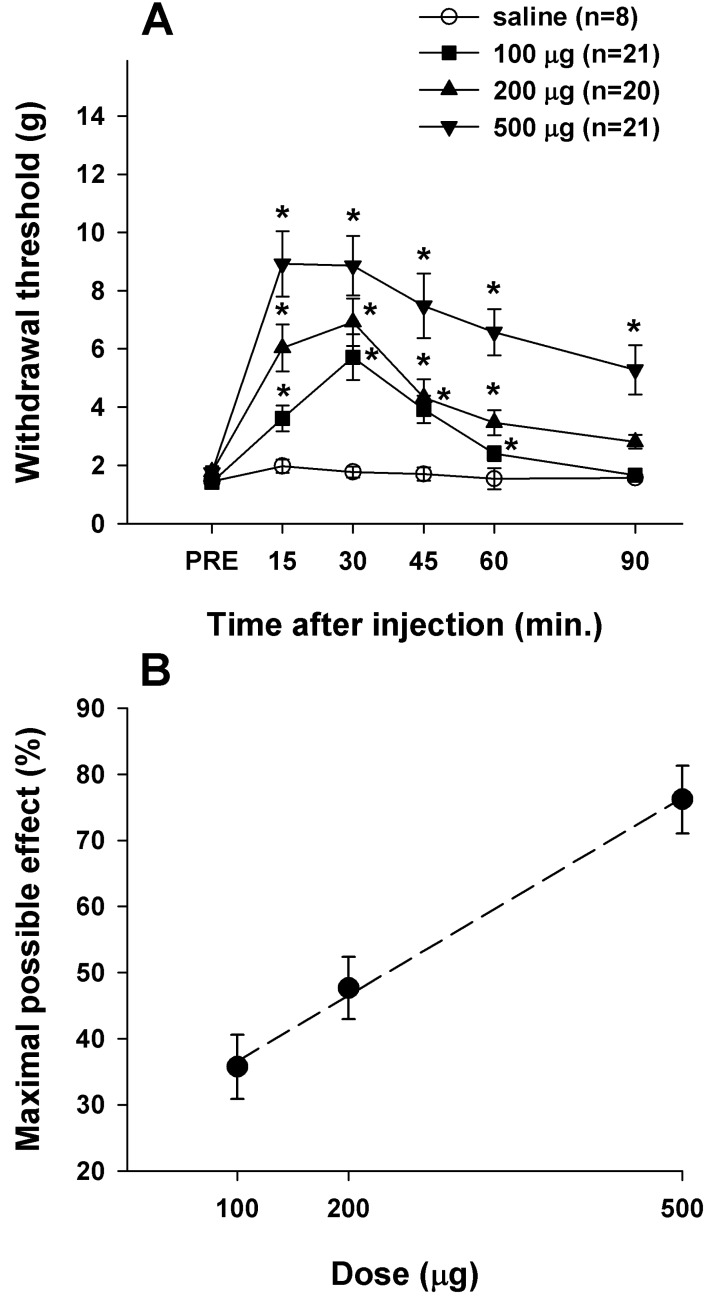
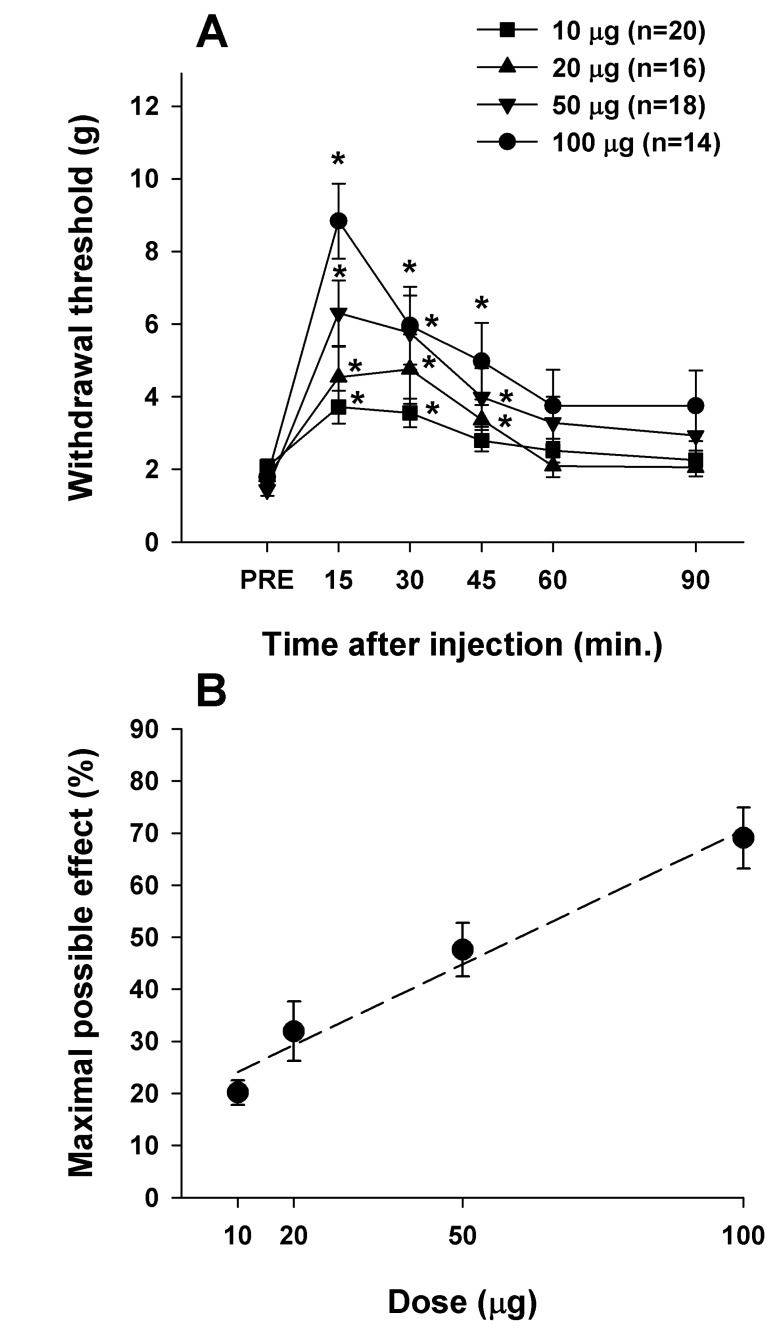
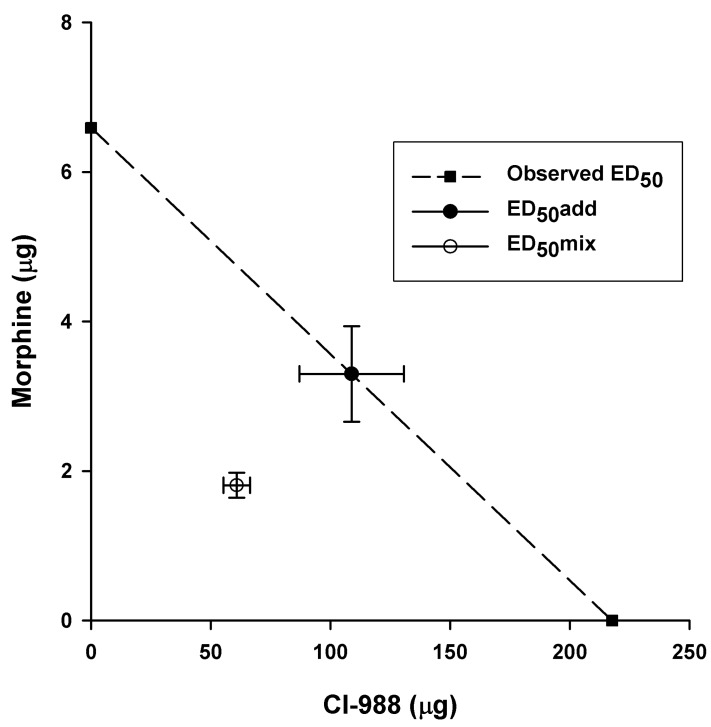
- Cholecystokinin is known to be involved in the modulation of nociception and to reduce the efficacy of morphine analgesia. This study investigated the effects of intrathecal administration of morphine and the cholecystokinin type B antagonist CI-988 on below-level neuropathic pain after spinal cord injury in rats. We also examined the interaction of morphine and CI-988 in the antinociceptive effect. Both morphine and CI-988 given individually increased the paw withdrawal threshold to mechanical stimulation in a dose-dependent manner. The combination of ineffective doses of intrathecally administered CI-988 and morphine produced significant analgesic effects and the combination of effective doses resulted in analgesic effects that were greater than the sum of the individual effects of each drug. Thus, morphine showed a synergistic interaction with CI-988 for analgesia of central neuropathic pain.
MeSH Terms
Figure
Reference
-
1. Siddall PJ, Molloy AR, Walker S, Mather LE, Rutkowski SB, Cousins MJ. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesth Analg. 2000; 91:1493–1498. PMID: 11094007.
Article2. Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009; 60:202–213. PMID: 19154757.
Article3. Portenoy RK, Foley KM, Inturrisi CE. The nature of opioid responsiveness and its implications for neuropathic pain: new hypotheses derived from studies of opioid infusions. Pain. 1990; 43:273–286. PMID: 1705692.
Article4. Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010; 150:573–581. PMID: 20705215.
Article5. Rowbotham MC, Hansson PT, Fields HL, Hill RG, Marchettini P. Efficacy of opioids. In : Hansson PT, editor. Neuropathic pain: Pathophysiology and treatment. Seattle: ISAP Press;2001. p. 203–213.6. Brewer KL, McMillan D, Nolan T, Shum K. Cortical changes in cholecystokinin mRNA are related to spontaneous pain behaviors following excitotoxic spinal cord injury in the rat. Brain Res Mol Brain Res. 2003; 118:171–174. PMID: 14559369.
Article7. Xu XJ, Puke MJ, Verge VM, Wiesenfeld-Hallin Z, Hughes J, Hökfelt T. Up-regulation of cholecystokinin in primary sensory neurons is associated with morphine insensitivity in experimental neuropathic pain in the rat. Neurosci Lett. 1993; 152:129–132. PMID: 8515864.
Article8. Kim J, Kim JH, Kim Y, Cho HY, Hong SK, Yoon YW. Role of spinal cholecystokinin in neuropathic pain after spinal cord hemisection in rats. Neurosci Lett. 2009; 462:303–307. PMID: 19619609.
Article9. Xu XJ, Hao JX, Seiger A, Hughes J, Hökfelt T, Wiesenfeld-Hallin Z. Chronic pain-related behaviors in spinally injured rats: evidence for functional alterations of the endogenous cholecystokinin and opioid systems. Pain. 1994; 56:271–277. PMID: 7912821.10. Coudoré-Civiale MA, Courteix C, Fialip J, Boucher M, Eschalier A. Spinal effect of the cholecystokinin-B receptor antagonist CI-988 on hyperalgesia, allodynia and morphine-induced analgesia in diabetic and mononeuropathic rats. Pain. 2000; 88:15–22. PMID: 11098095.
Article11. Torres-López JE, Juárez-Rojop IE, Granados-Soto V, Diaz-Zagoya JC, Flores-Murrieta FJ, Ortíz-López JU, Cruz-Vera J. Peripheral participation of cholecystokinin in the morphine-induced peripheral antinociceptive effect in non-diabetic and diabetic rats. Neuropharmacology. 2007; 52:788–795. PMID: 17157334.
Article12. Idänpään-Heikkilä JJ, Perrot S, Guilbaud G, Kayser V. In mononeuropathic rats, the enhancement of morphine antinociception by L-365,260, a selective CCK(B) receptor antagonist, depends on the dose of systemic morphine and stimulus characteristics. Eur J Pharmacol. 1997; 325:155–164. PMID: 9163562.
Article13. Agnes RS, Ying J, Kövér KE, Lee YS, Davis P, Ma SW, Badghisi H, Porreca F, Lai J, Hruby VJ. Structure-activity relationships of bifunctional cyclic disulfide peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. Peptides. 2008; 29:1413–1423. PMID: 18502541.
Article14. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–462. PMID: 7387124.
Article15. Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985; 88:123–134. PMID: 3979506.
Article16. Kim J, Jung JI, Na HS, Hong SK, Yoon YW. Effects of morphine on mechanical allodynia in a rat model of central neuropathic pain. Neuroreport. 2003; 14:1017–1020. PMID: 12802194.
Article17. Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001; 298:865–872. PMID: 11504778.18. Tallarida RJ, Stone DJ Jr, Raffa RB. Efficient designs for studying synergistic drug combinations. Life Sci. 1997; 61:PL 417–PL 425.
Article19. Kim J, Yoon YW, Hong SK, Na HS. Cold and mechanical allodynia in both hindpaws and tail following thoracic spinal cord hemisection in rats: time courses and their correlates. Neurosci Lett. 2003; 343:200–204. PMID: 12770696.
Article20. Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005; 117:77–87. PMID: 16098668.21. Hebb AL, Poulin JF, Roach SP, Zacharko RM, Drolet G. Cholecystokinin and endogenous opioid peptides: interactive influence on pain, cognition, and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2005; 29:1225–1238. PMID: 16242828.
Article22. Wu HE, Schwasinger ET, Hong JS, Tseng LF. Pretreatment with antiserum against dynorphin, substance P, or cholecystokinin enhances the morphine-produced anti-allodynia in the sciatic nerve ligated mice. Neurosci Lett. 2005; 386:46–51. PMID: 15982809.
Article23. Xu XJ, Alster P, Wu WP, Hao JX, Wiesenfeld-Hallin Z. Increased level of cholecystokinin in cerebrospinal fluid is associated with chronic pain-like behavior in spinally injured rats. Peptides. 2001; 22:1305–1308. PMID: 11457525.
Article24. Wiesenfeld-Hallin Z, Xu XJ. The role of cholecystokinin in nociception, neuropathic pain and opiate tolerance. Regul Pept. 1996; 65:23–28. PMID: 8876032.
Article25. Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000; 87:265–273. PMID: 10963906.
Article26. Verge VM, Wiesenfeld-Hallin Z, Hökfelt T. Cholecystokinin in mammalian primary sensory neurons and spinal cord: in situ hybridization studies in rat and monkey. Eur J Neurosci. 1993; 5:240–250. PMID: 8261105.27. Cesselin F. Opioid and anti-opioid peptides. Fundam Clin Pharmacol. 1995; 9:409–433. PMID: 8617406.
Article28. Mollereau C, Roumy M, Zajac JM. Opioid-modulating peptides: mechanisms of action. Curr Top Med Chem. 2005; 5:341–355. PMID: 15857316.
Article29. Zhou Y, Sun YH, Zhang ZW, Han JS. Increased release of immunoreactive cholecystokinin octapeptide by morphine and potentiation of mu-opioid analgesia by CCKB receptor antagonist L-365,260 in rat spinal cord. Eur J Pharmacol. 1993; 234:147–154. PMID: 8387008.30. Stanfa LC, Dickenson AH. Cholecystokinin as a factor in the enhanced potency of spinal morphine following carrageenin inflammation. Br J Pharmacol. 1993; 108:967–973. PMID: 8485635.
Article31. Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012; 7:CD008943. PMID: 22786518.
Article32. Hanlon KE, Herman DS, Agnes RS, Largent-Milnes TM, Kumarasinghe IR, Ma SW, Guo W, Lee YS, Ossipov MH, Hruby VJ, Lai J, Porreca F, Vanderah TW. Novel peptide ligands with dual acting pharmacophores designed for the pathophysiology of neuropathic pain. Brain Res. 2011; 1395:1–11. PMID: 21550594.
Article33. McCleane G. The cholecystokinin antagonist proglumide has an analgesic effect when used alone in human neuropathic pain: A case report. Pain Clinic. 2003; 15:71–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synergistic anti-allodynic effect between intraperitoneal thalidomide and morphine on rat spinal nerve ligation-induced neuropathic pain
- The Treatment of Central Pain after Spinal Cord Injury
- Effects of cyanocobalamin and its combination with morphine on neuropathic rats and the relationship between these effects and thrombospondin-4 expression
- The Antiallodynic Effect and the Change of the alpha2 Adrenergic Receptor Subtype mRNA Expression by Morphine Administration in a Spinal Nerve Ligation Rat Model
- Interaction between Neostigmine and Morphine in the Neuropathic Pain Model