Korean J Physiol Pharmacol.
2014 Oct;18(5):431-439. 10.4196/kjpp.2014.18.5.431.
Inhibitory Effects of Ginsenoside-Rb2 on Nicotinic Stimulation-Evoked Catecholamine Secretion
- Affiliations
-
- 1Department of Internal Medicine (Division of Pulmonary and Critical Care Medicine), Veterans Health Service Medical Center, Seoul 134-791, Korea.
- 2Department of Anesthesiology and Pain Medicine, School of Medicine, Chosun University, Gwangju 501-759, Korea.
- 3Department of Pharmacology, School of Medicine, Chosun University, Gwangju 501-759, Korea. dylim@chosun.ac.kr
- KMID: 2285543
- DOI: http://doi.org/10.4196/kjpp.2014.18.5.431
Abstract
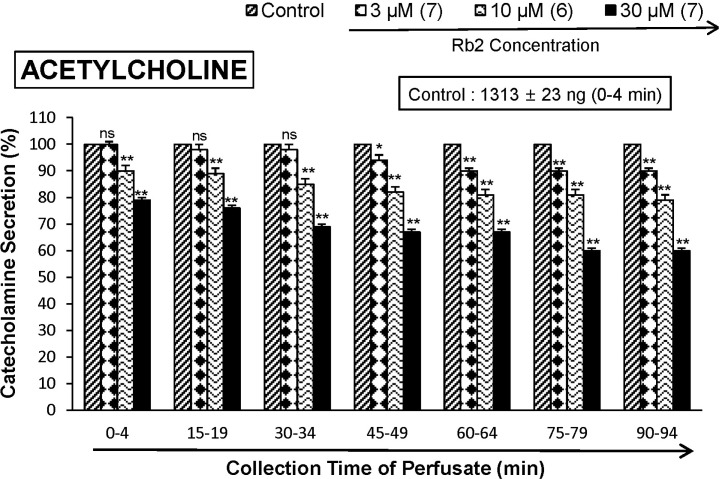
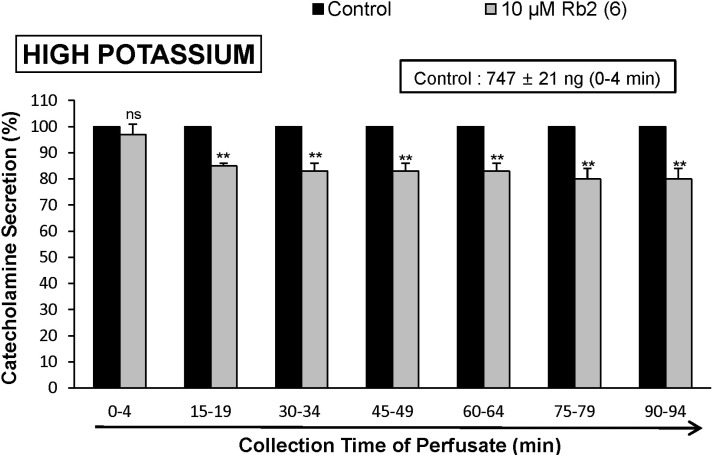
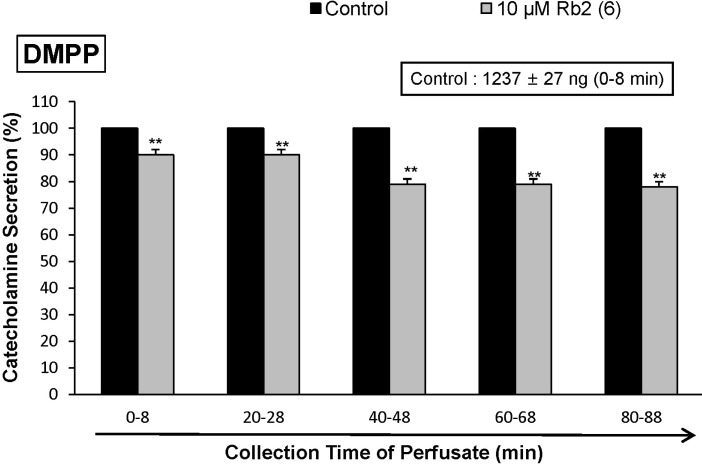
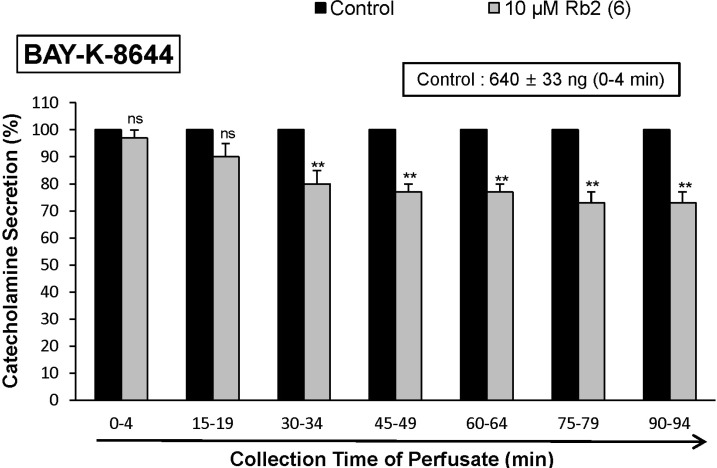
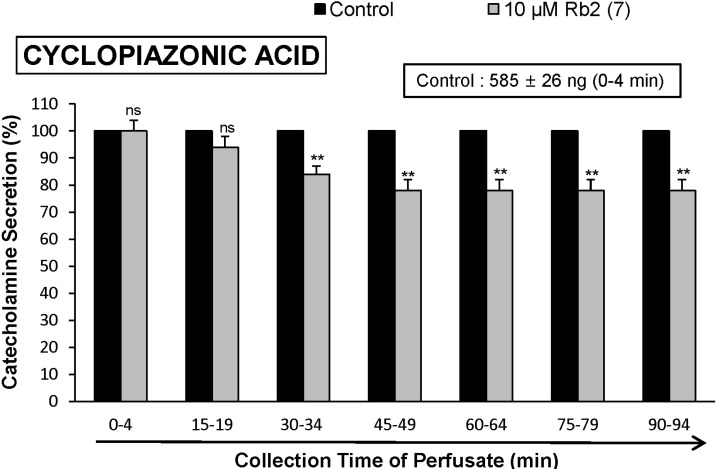
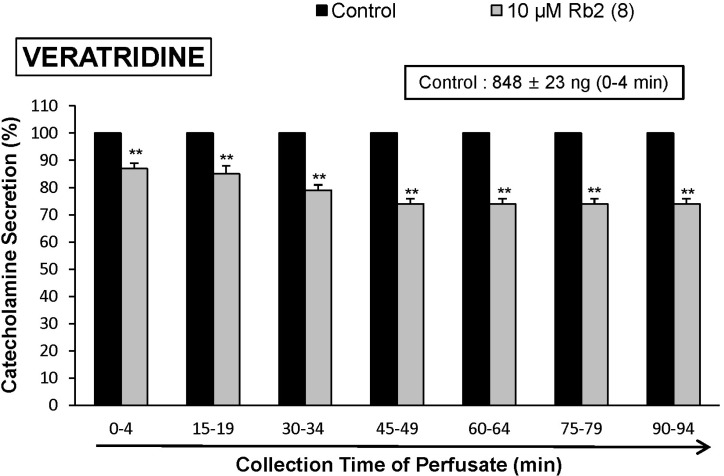
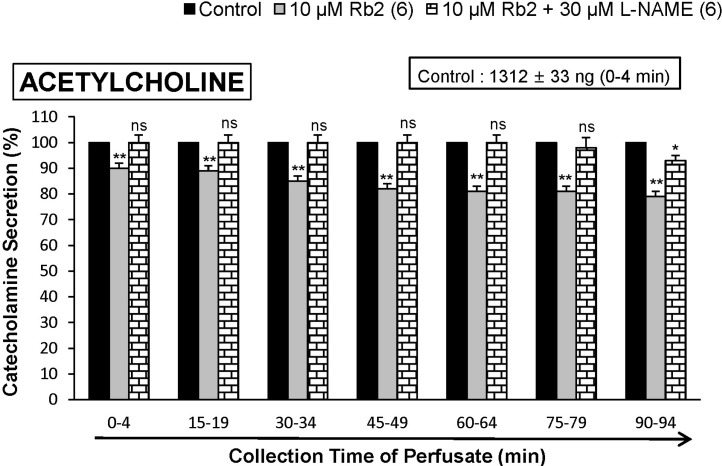
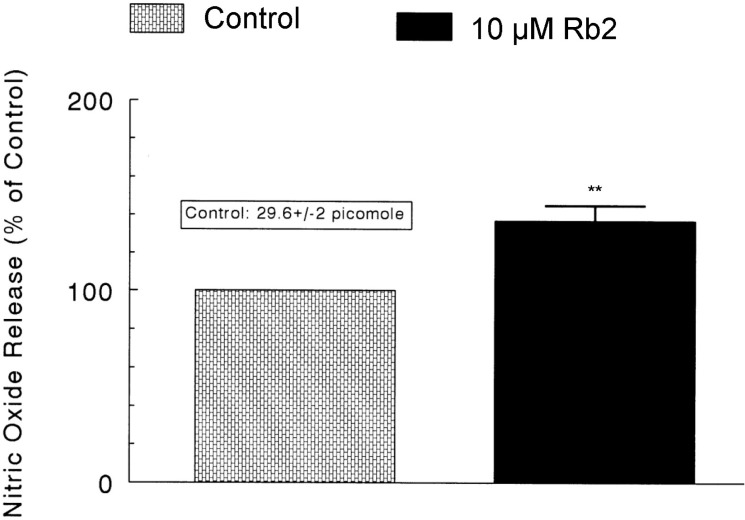
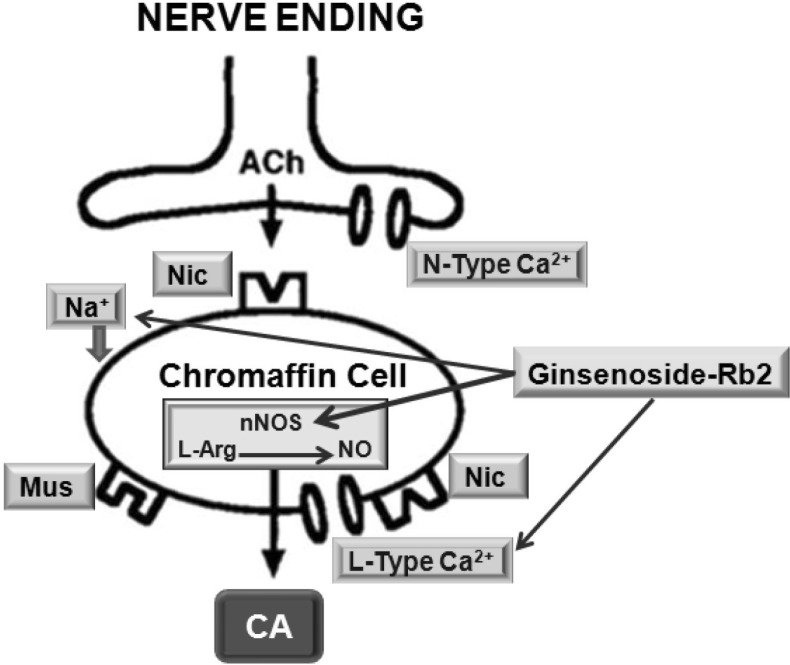
- The aim of the present study was to investigate whether ginsenoside-Rb2 (Rb2) can affect the secretion of catecholamines (CA) in the perfused model of the rat adrenal medulla. Rb2 (3~30 microM), perfused into an adrenal vein for 90 min, inhibited ACh (5.32 mM)-evoked CA secretory response in a dose- and time-dependent fashion. Rb2 (10 microM) also time-dependently inhibited the CA secretion evoked by DMPP (100 microM, a selective neuronal nicotinic receptor agonist) and high K+ (56 mM, a direct membrane depolarizer). Rb2 itself did not affect basal CA secretion (data not shown). Also, in the presence of Rb2 (50 microg/mL), the secretory responses of CA evoked by veratridine (a selective Na+ channel activator (50 microM), Bay-K-8644 (an L-type dihydropyridine Ca2+ channel activator, 10 microM), and cyclopiazonic acid (a cytoplasmic Ca2+-ATPase inhibitor, 10 microM) were significantly reduced, respectively. Interestingly, in the simultaneous presence of Rb2 (10 microM) and L-NAME (an inhibitor of NO synthase, 30 microM), the inhibitory responses of Rb2 on ACh-evoked CA secretory response was considerably recovered to the extent of the corresponding control secretion compared with the inhibitory effect of Rb2-treatment alone. Practically, the level of NO released from adrenal medulla after the treatment of Rb2 (10 microM) was greatly elevated compared to the corresponding basal released level. Collectively, these results demonstrate that Rb2 inhibits the CA secretory responses evoked by nicotinic stimulation as well as by direct membrane-depolarization from the isolated perfused rat adrenal medulla. It seems that this inhibitory effect of Rb2 is mediated by inhibiting both the influx of Ca2+ and Na+ into the adrenomedullary chromaffin cells and also by suppressing the release of Ca2+ from the cytoplasmic calcium store, at least partly through the increased NO production due to the activation of nitric oxide synthase, which is relevant to neuronal nicotinic receptor blockade.
Keyword
MeSH Terms
-
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Adrenal Medulla
Animals
Calcium
Catecholamines
Chromaffin Cells
Cytoplasm
Dimethylphenylpiperazinium Iodide
Membranes
Neurons
NG-Nitroarginine Methyl Ester
Nitric Oxide Synthase
Rats
Receptors, Nicotinic
Veins
Veratridine
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Calcium
Catecholamines
Dimethylphenylpiperazinium Iodide
NG-Nitroarginine Methyl Ester
Nitric Oxide Synthase
Receptors, Nicotinic
Veratridine
Figure
Reference
-
1. Lim DY, Park KB, Kim KY, Lee KS, Moon JK, Kim YH. Influence to total ginseng saponin on secretion of catecholamines in the isolated adrenal gland of rabbits. Korean Biochem J. 1987; 20:230–238.2. Lim DY, Park KB, Kim KH, Choi CH, Bae JW, Kim MW. Studies on secretion of catecholamines evoked by panaxadiol in the isolated rabbit adrenal gland. Korean J Pharmacol. 1988; 24:31–42.3. Lim DY, Choi CH, Kim CD, Kim KH, Kim SB, Lee BJ, Chung MH. Influnce of Panaxatriol-type saponin on secretion of catecholamine from isolated perfused rabbit adrenal gland. Arch Pharm Res. 1989; 12:166–175.4. Hong SP, Choi H, Cho SH, Lee YK, Woo SC, Kim IS, Oh SH, Yang WH, Lim DY. Influence of total Ginseng saponin on nicotinic stimulation-induced catecholamine secretion from the perfused rat adrenal gland. J Korean Soc Hypertens. 1999; 5:159–168.5. Kudo K, Akasaka Y, Miyate Y, Takahashi E, Tachikawa E, Kashimoto T. Effects of red ginseng fractions on catecholamine secretion from bovine adrenal medullary cells. J Med Pharm Soc WAKAN-YAKU. 1992; 9:236–239.6. Tachikawa E, Kudo K, Kashimoto T, Takahashi E. Ginseng saponins reduce acetylcholine-evoked Na+ influx and catecholamine secretion in bovine adrenal chromaffin cells. J Pharmacol Exp Ther. 1995; 273:629–636. PMID: 7752064.7. Kudo K, Tachikawa E, Kashimoto T, Takahashi E. Properties of ginseng saponin inhibition of catecholamine secretion in bovine adrenal chromaffin cells. Eur J Pharmacol. 1998; 341:139–144. PMID: 9543231.
Article8. Tachikawa E, Kudo K, Nunokawa M, Kashimoto T, Takahashi E, Kitagawa S. Characterization of ginseng saponin ginsenoside-Rg(3) inhibition of catecholamine secretion in bovine adrenal chromaffin cells. Biochem Pharmacol. 2001; 62:943–951. PMID: 11543730.9. Jeon BH, Kim CS, Kim HS, Park JB, Nam KY, Chang SJ. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000; 21:1095–1100. PMID: 11603282.10. Kim ND, Kim EM, Kang KW, Cho MK, Choi SY, Kim SG. Ginsenoside Rg3 inhibits phenylephrine-induced vascular contraction through induction of nitric oxide synthase. Br J Pharmacol. 2003; 140:661–670. PMID: 14534150.
Article11. Han K, Shin IC, Choi KJ, Yun YP, Hong JT, Oh KW. Korea red ginseng water extract increases nitric oxide concentrations in exhaled breath. Nitric Oxide. 2005; 12:159–162. PMID: 15797844.
Article12. Kim ND, Kang SY, Schini VB. Ginsenosides evoke endothelium-dependent vascular relaxation in rat aorta. Gen Pharmacol. 1994; 25:1071–1077. PMID: 7875528.
Article13. Kim ND, Kang SY, Park JH, Schini-Kerth VB. Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta: role of K+ channels. Eur J Pharmacol. 1999; 367:41–49. PMID: 10082263.14. Kang SY, Schini-Kerth VB, Kim ND. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta. Life Sci. 1995; 56:1577–1586. PMID: 7723586.
Article15. Hien TT, Kim ND, Pokharel YR, Oh SJ, Lee MY, Kang KW. Ginsenoside Rg3 increases nitric oxide production via increases in phosphorylation and expression of endothelial nitric oxide synthase: essential roles of estrogen receptor-dependent PI3-kinase and AMP-activated protein kinase. Toxicol Appl Pharmacol. 2010; 246:171–183. PMID: 20546771.
Article16. Leung KW, Cheng YK, Mak NK, Chan KK, Fan TP, Wong RN. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006; 580:3211–3216. PMID: 16696977.
Article17. Han SW, Kim H. Ginsenosides stimulate endogenous production of nitric oxide in rat kidney. Int J Biochem Cell Biol. 1996; 28:573–580. PMID: 8697102.
Article18. Chai H, Zhou W, Lin P, Lumsden A, Yao Q, Chen C. Ginsenosides block HIV protease inhibitor ritonavir-induced vascular dysfunction of porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2005; 288:H2965–H2971. PMID: 15681703.
Article19. Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981; 313:463–480. PMID: 7277230.
Article20. Anton AH, Sayre DF. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962; 138:360–375. PMID: 14013351.21. McVeigh GE, Hamilton P, Wilson M, Hanratty CG, Leahey WJ, Devine AB, Morgan DG, Dixon LJ, McGrath LT. Platelet nitric oxide and superoxide release during the development of nitrate tolerance: effect of supplemental ascorbate. Circulation. 2002; 106:208–213. PMID: 12105160.22. Tallarida RJ, Murray RB. Manual of pharmacologic calculation with computer programs. 2nd ed. New York: Speringer-Verlag;1987. p. 132.23. García AG, Sala F, Reig JA, Viniegra S, Frías J, Fontériz R, Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984; 309:69–71. PMID: 6201747.
Article24. Lim DY, Kim CD, Ahn GW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 1992; 15:115–125.
Article25. Goeger DE, Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989; 38:3995–4003. PMID: 2532015.26. Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989; 264:17816–17823. PMID: 2530215.27. Wada Y, Satoh K, Taira N. Cardiovascular profile of Bay K 8644, a presumed calcium channel activator, in the dog. Naunyn Schmiedebergs Arch Pharmacol. 1985; 328:382–387. PMID: 2581147.
Article28. Torres M, Ceballos G, Rubio R. Possible role of nitric oxide in catecholamine secretion by chromaffin cells in the presence and absence of cultured endothelial cells. J Neurochem. 1994; 63:988–996. PMID: 7519669.
Article29. Uchiyama Y, Morita K, Kitayama S, Suemitsu T, Minami N, Miyasako T, Dohi T. Possible involvement of nitric oxide in acetylcholine-induced increase of intracellular Ca2+ concentration and catecholamine release in bovine adrenal chromaffin cells. Jpn J Pharmacol. 1994; 65:73–77. PMID: 8089933.30. O'Sullivan AJ, Burgoyne RD. Cyclic GMP regulates nicotineinduced secretion from cultured bovine adrenal chromaffin cells: effects of 8-bromo-cyclic GMP, atrial natriuretic peptide, and nitroprusside (nitric oxide). J Neurochem. 1990; 54:1805–1808. PMID: 2157818.31. Breslow MJ, Tobin JR, Bredt DS, Ferris CD, Snyder SH, Traystman RJ. Role of nitric oxide in adrenal medullary vasodilation during catecholamine secretion. Eur J Pharmacol. 1992; 210:105–106. PMID: 1376270.
Article32. Breslow MJ, Tobin JR, Bredt DS, Ferris CD, Snyder SH, Traystman RJ. Nitric oxide as a regulator of adrenal blood flow. Am J Physiol. 1993; 264:H464–H469. PMID: 7680537.
Article33. Viveros OH. Mechanism of secretion of catecholaminies from adrenal medulla. In : Blaschko H, Sayers G, Smith DA, editors. Handbook of physiology, Endocrinology. Vol VI, Sect 7, The adrenal gland. Washington DC: American physiological society;1975. p. 389–426.34. Han KH, Choe SC, Kim HS, Sohn DW, Nam KY, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Effect of red ginseng on blood pressure in patients with essential hypertension and white coat hypertension. Am J Chin Med. 1998; 26:199–209. PMID: 9799972.
Article35. Jeon BH, Kim CS, Park KS, Lee JW, Park JB, Kim KJ, Kim SH, Chang SJ, Nam KY. Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen Pharmacol. 2000; 35:135–141. PMID: 11744235.
Article36. Sung J, Han KH, Zo JH, Park HJ, Kim CH, Oh BH. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med. 2000; 28:205–216. PMID: 10999439.
Article37. Dixon WR, Garcia AG, Kirpekar SM. Release of catecholamines and dopamine beta-hydroxylase from the perfused adrenal gland of the cat. J Physiol. 1975; 244:805–824. PMID: 1133780.
Article38. Viveros OH, Arqueros L, Kirshner N. Release of catecholamines and dopamine beta-hydroxylase from the adrenal medulla. Life Sci. 1968; 7:609–618.39. Douglas WW. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968; 34:451–474. PMID: 4882190.
Article40. Sorimachi M, Yoshida K. Exocytotic release of catecholamines and dopamine-beta-hydroxylase from the perfused adrenal gland of the rabbit and cat. Br J Pharmacol. 1979; 65:117–125. PMID: 760886.41. Nakazato Y, Ohga A, Oleshansky M, Tomita U, Yamada Y. Voltage-independent catecholamine release mediated by the activation of muscarinic receptors in guinea-pig adrenal glands. Br J Pharmacol. 1988; 93:101–109. PMID: 3349226.
Article42. Lim DY, Hwang DH. Studies on secretion of catecholamines evoked by DMPP and McN-A-343 in the rat adrenal gland. Korean J Pharmacol. 1991; 27:53–67.43. Rang HP, Colquhoun D, Rang HP. The action of ganglionic blocking drugs on the synaptic responses of rat submandibular ganglion cells. Br J Pharmacol. 1982; 75:151–168. PMID: 6122479.
Article44. Weaver WR, Wolf KM, Chiappinelli VA. Functional heterogeneity of nicotinic receptors in the avian lateral spiriform nucleus detected with trimethaphan. Mol Pharmacol. 1994; 46:993–1001. PMID: 7969091.45. Wada A, Yanagihara N, Izumi F, Sakurai S, Kobayashi H. Trifluoperazine inhibits 45Ca2+ uptake and catecholamine secretion and synthesis in adrenal medullary cells. J Neurochem. 1983; 40:481–486. PMID: 6401801.46. Schramm M, Thomas G, Towart R, Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983; 303:535–537. PMID: 6190088.47. Fisher SK, Holz RW, Agranoff BW. Muscarinic receptors in chromaffin cell cultures mediate enhanced phospholipid labeling but not catecholamine secretion. J Neurochem. 1981; 37:491–497. PMID: 7264672.
Article48. Yanagihara N, Isosaki M, Ohuchi T, Oka M. Muscarinic receptor-mediated increase in cyclic GMP level in isolated bovine adrenal medullary cells. FEBS Lett. 1979; 105:296–298. PMID: 226413.
Article49. Wakade AR, Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983; 10:973–978. PMID: 6139771.
Article50. Kilpatrick DL, Slepetis R, Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 1981; 36:1245–1255. PMID: 6259284.
Article51. Kilpatrick DL, Slepetis RJ, Corcoran JJ, Kirshner N. Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem. 1982; 38:427–435. PMID: 7108549.
Article52. Knight DE, Kesteven NT. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983; 218:177–199. PMID: 6135214.53. Wada A, Takara H, Izumi F, Kobayashi H, Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medulla cells. Neuroscience. 1985; 15:283–292. PMID: 2409474.
Article54. Kidokoro Y, Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980; 307:199–216. PMID: 7205664.
Article55. Burgoyne RD. Mechanisms of secretion from adrenal chromaffin cells. Biochim Biophys Acta. 1984; 779:201–216. PMID: 6234026.
Article56. Oka M, Isosaki M, Yanagihara N. Isolated bovine adrenal medullary cells: studies on regulation of catecholamine synthesis and release. In : Usdin E, Kopin IJ, Brachas J, editors. Catecholamines: basic and clinical frontiers. Oxford: Pergamon Press;1979. p. 70–72.57. Suzuki M, Muraki K, Imaizumi Y, Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 1992; 107:134–140. PMID: 1330156.58. Uyama Y, Imaizumi Y, Watanabe M. Effects of cyclopiazonic acid, a novel Ca2+-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 1992; 106:208–214. PMID: 1387024.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of glucocorticoids on cholinergic stimulation-induced catecholamine secretion from the rat adrenal medulla
- Naltrexone Inhibits Catecholamine Secretion Evoked by Nicotinic Receptor Stimulation in the Perfused Rat Adrenal Medulla
- Influence of Tacrine on Catecholamine Secretion in the Perfused Rat Adrenal Gland
- Influence of Ketamine on Catecholamine Secretion in the Perfused Rat Adrenal Medulla
- Resveratrol Inhibits Nicotinic Stimulation-Evoked Catecholamine Release from the Adrenal Medulla