Korean J Physiol Pharmacol.
2014 Oct;18(5):365-369. 10.4196/kjpp.2014.18.5.365.
Atypical Antidepressant Activity of 3,4-Bis(3,4-Dimethoxyphenyl) Furan-2,5-Dione Isolated from Heart Wood of Cedrus deodara, in Rodents
- Affiliations
-
- 1Manipal College of Pharmaceutical Sciences, Manipal University, Manipal 576104, Karnataka, India. mailnandakumar77@gmail.com
- 2Farooqia College of Pharmacy, Mysore 570021, Karnataka, India.
- 3Department of Pharmaceutical Technology, Anna University Chennai, Regional Office, Tiruchirappalli 620 024, Tamil Nadu, India. puratchipharma@gmail.com
- KMID: 2285533
- DOI: http://doi.org/10.4196/kjpp.2014.18.5.365
Abstract
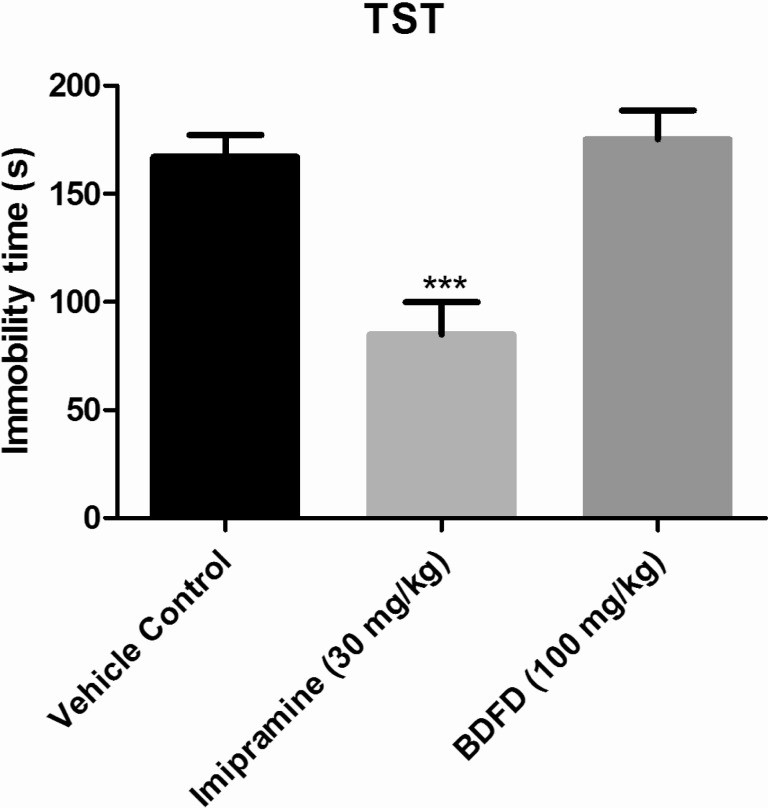
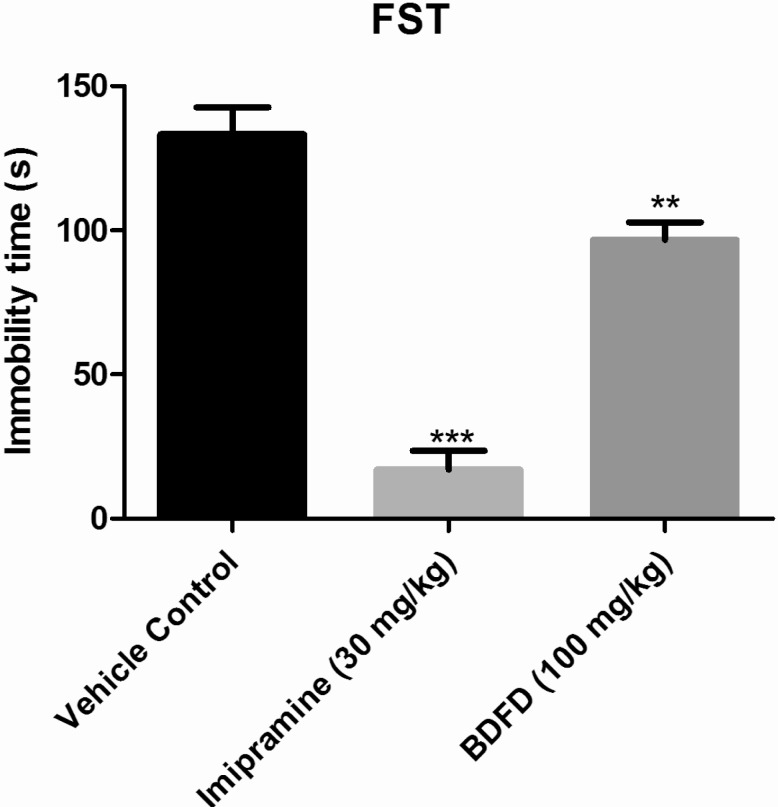
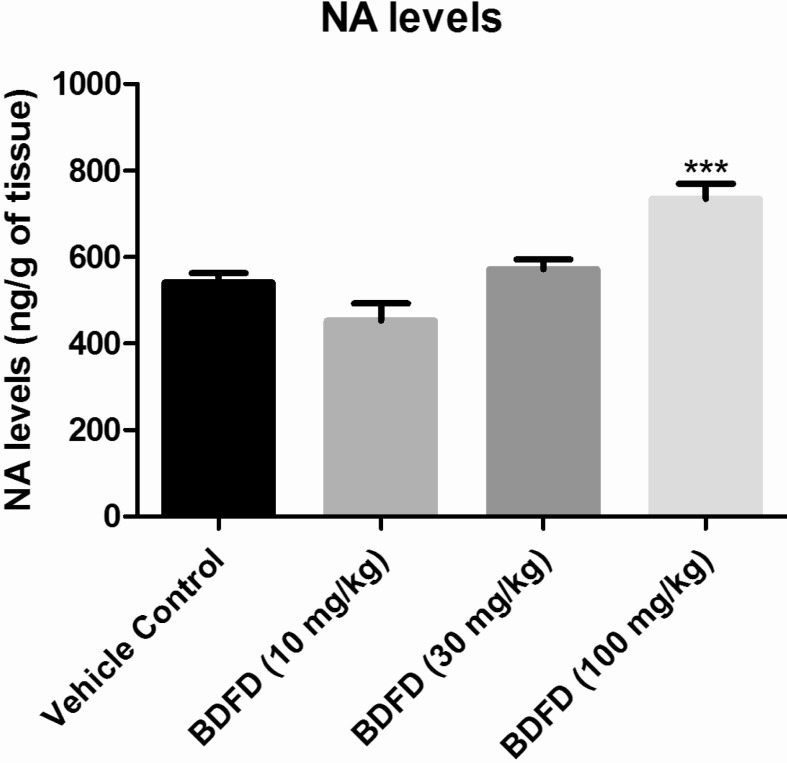
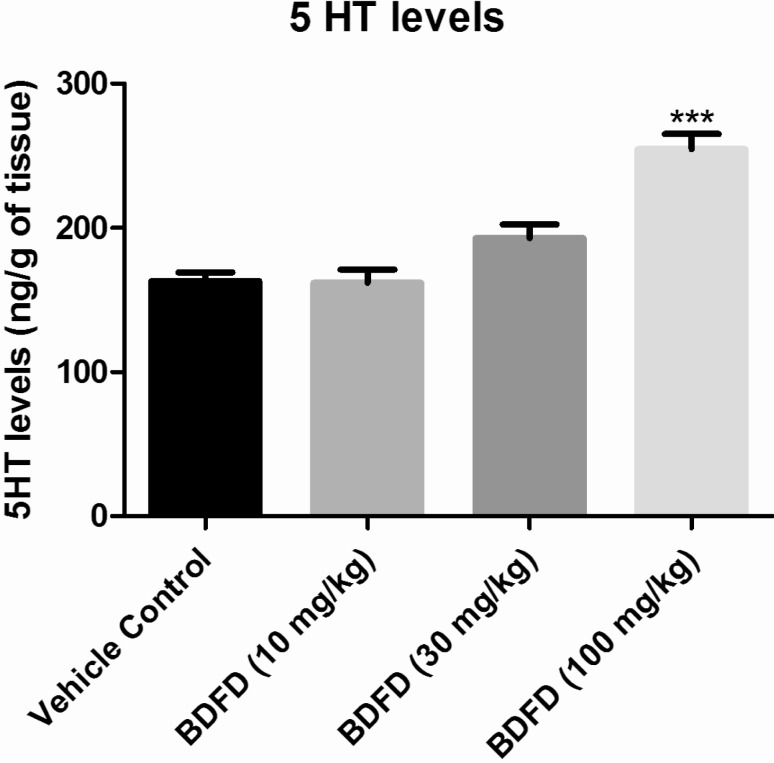
- Cedrus deodara (Pinaceae) has been used traditionally in Ayurveda for the treatment of central nervous system disorders. 3,4-bis(3,4-dimethoxyphenyl)furan-2,5-dione (BDFD) was isolated from heart wood of Cedrus deodara and was shown to have antiepileptic and anxiolytic activity. Thus, the present study was aimed to explore its anti-depressant effect and to correlate the effect with serotonin and nor adrenaline levels of brain. Albino mice were used as experimental animal. Animals were divided in to three groups; vehicle control, imipramine (30 mg/kg i.p.), BDFD (100 mg/kg i.p.). Tail suspension test (TST) and forced swim test (FST) was performed to evaluate antidepressant effect of BDFD. BDFD (100 mg/kg, i.p.) showed a significant decrease in immobility time when subjected to FST whereas immobility time was not significantly altered in TST. BDFD treatment increased serotonin and noradrenaline levels in the brain which is indicative of BDFD having possible atypical antidepressant action.
Keyword
MeSH Terms
Figure
Reference
-
1. Kar K, Puri VN, Patnaik GK, Sur RN, Dhawan BN, Kulshrestha DK, Rastogi RP. Spasmolytic constituents of Cedrus deodara (Roxb.) Loud: pharmacological evaluation of himachalol. J Pharm Sci. 1975; 64:258–262. PMID: 47907.
Article2. Shinde U, Phadke A, Nair A, Mungantiwar A, Dikshit V, Saraf M. Preliminary studies on the immunomodulatory activity of Cedrus deodara wood oil. Fitoterapia. 1999; 70:333–339.
Article3. Saxena A, Saxena AK, Singh J, Bhushan S. Natural antioxidants synergistically enhance the anticancer potential of AP9-cd, a novel lignan composition from Cedrus deodara in human leukemia HL-60 cells. Chem Biol Interact. 2010; 188:580–590. PMID: 20932957.
Article4. Dikshit A, Dixit S. Cedrus oil--a promising antifungal agent. Indian Perfumer. 1982; 26:216–227.5. Bisht LS, Brindavanam NB, Kimothic P. Comparative study of herbal agents used for fumigtation in relations to formulation(*). Anc Sci Life. 1988; 8:125–132. PMID: 22557643.6. Phillipson JD, Anderson LA. Ethnopharmacology and Western medicine. J Ethnopharmacol. 1989; 25:61–72. PMID: 2654491.
Article7. Gupta PP, Kulshrestha DK, Patnaik GK. Antiallergic activity of cedrus deodara. J Med Aromatic Plant Sci. 1997; 19:1007–1008.8. Tiwari AK, Srinivas PV, Kumar SP, Rao JM. Free radical scavenging active components from Cedrus deodara. J Agric Food Chem. 2001; 49:4642–4645. PMID: 11600001.
Article9. Nisha M, Kalyanasundaram M, Paily KP, Abidha , Vanamail P, Balaraman K. In vitro screening of medicinal plant extracts for macrofilaricidal activity. Parasitol Res. 2007; 100:575–579. PMID: 17013649.
Article10. Singh A, Singh DK. Effect of herbal molluscicides and their combinations on the reproduction of the snail Lymnaea acuminata. Arch Environ Contam Toxicol. 2004; 46:470–477. PMID: 15253044.
Article11. Shivanand P, Viral D, Manish G, Subhash V, Jaganathan K. Formulation and evaluation of cedrus deodara loud extract. Int J Chem Tech Res. 2009; 100:1145–1152.12. Gautam R, Saklani A, Jachak SM. Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol. 2007; 110:200–234. PMID: 17276637.
Article13. Ramesh C, Krishnadas N, Radhakrishnan R, Rangappa S, Viswanatha GLS, Rajesh D, Gopal M, Talwar S. Anti-urolithiatic activity of heart wood extract of cedrus deodara in rats. J Complement Integr Med. 2010; 7:1–9.
Article14. Kumar A, Singh V, Chaudhary AK. Gastric antisecretory and antiulcer activities of Cedrus deodara (Roxb.) Loud. in Wistar rats. J Ethnopharmacol. 2011; 134:294–297. PMID: 21182918.
Article15. Dhayabaran D, Florance EJ, Nandakumar K, Shanmugarathinam A, Puratchikody A. Anticonvulsant activity of fraction isolated from ethanolic extract of heartwood of Cedrus deodara. J Nat Med. 2014; 68:310–315. PMID: 23959538.
Article16. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985; 85:367–370. PMID: 3923523.
Article17. Lee JK. Anti-depressant like effect of methyl gallate isolated from acer barbinerve in mice. Korean J Physiol Pharmacol. 2013; 17:441–446. PMID: 24227946.18. Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl). 2005; 177:245–255. PMID: 15609067.
Article19. Lakshmana MK, Raju TR. An isocratic assay for norepinephrine, dopamine, and 5-hydroxytryptamine using their native fluorescence by high-performance liquid chromatography with fluorescence detection in discrete brain areas of rat. Anal Biochem. 1997; 246:166–170. PMID: 9073352.
Article20. Akiskal HS, Van Valkenburg C. Mood disorders. Diagnostic Interviewing. Springer;1994. p. 79–107.21. Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007; 12:331–359. PMID: 17389902.
Article22. Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007; 24:495–517. PMID: 17117412.
Article23. Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000; 47:305–313. PMID: 10686265.
Article24. Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974; 36:248–257. PMID: 4829619.
Article25. Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011; 7:121–147. PMID: 21225412.
Article26. Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. Effect of Berberine on Depression- and Anxiety-Like Behaviors and Activation of the Noradrenergic System Induced by Development of Morphine Dependence in Rats. Korean J Physiol Pharmacol. 2012; 16:379–386. PMID: 23269899.
Article27. Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl). 2005; 177:245–255. PMID: 15609067.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of White-rot Fungi for Biopulping of Wood
- Animal Models of Depresstion and Screening of Antidepressants
- Dermatophytes and Keratinophilic Fungi Isolated from Wild Rodents in Korea
- In Vitro Effect of Carbamazepine, D-(-)-2-Amino-5-Phosphonopentanoic Acid and 6-Cyano-7-Nitroquinoxaline-Dione on Pentylenetetrazole-Induced Epileptiform Activity in Young Rat Visual Cortex
- The Changes of BIS and Hemodynamic Variables during Pediatric Open Heart Surgery with Sevoflurane or Midazolam