Korean J Physiol Pharmacol.
2012 Dec;16(6):387-392. 10.4196/kjpp.2012.16.6.387.
Antinociceptive Effect of Cyperi rhizoma and Corydalis tuber Extracts on Neuropathic Pain in Rats
- Affiliations
-
- 1Department of Physiology and Brain Research Institute, Chungnam National University Medical School, Daejeon 301-747, Korea. kim0827@cnu.ac.kr
- 2Department of Veterinary Physiology, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea.
- 3Department of Maxillofacial Tissue Regeneration, School of Dentistry, Kyung Hee University, Seoul 130-701, Korea.
- 4Laboratory of Molecular Signal Transduction, Center for Neural Science, Korea Institute of Science and Technology (KIST), Seoul 136-791, Korea.
- KMID: 2285446
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.387
Abstract
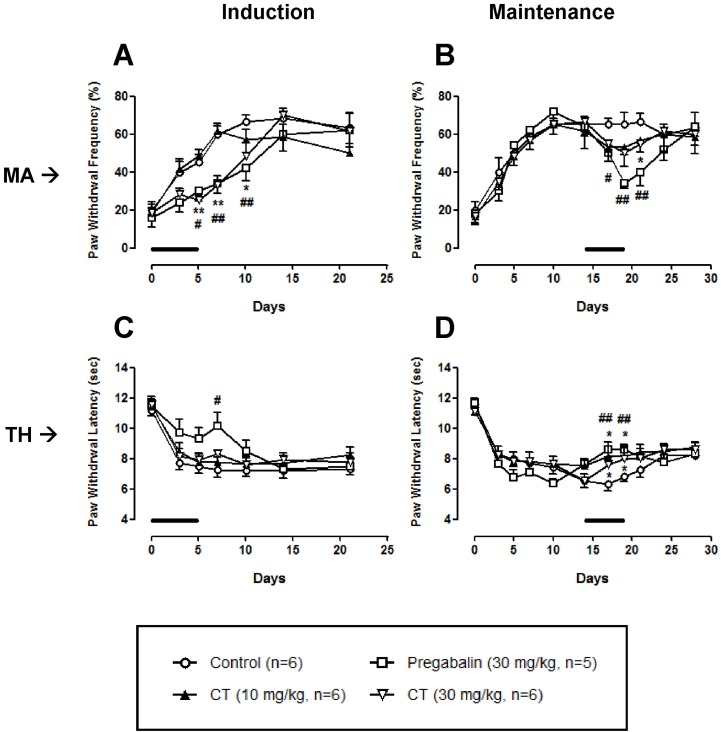
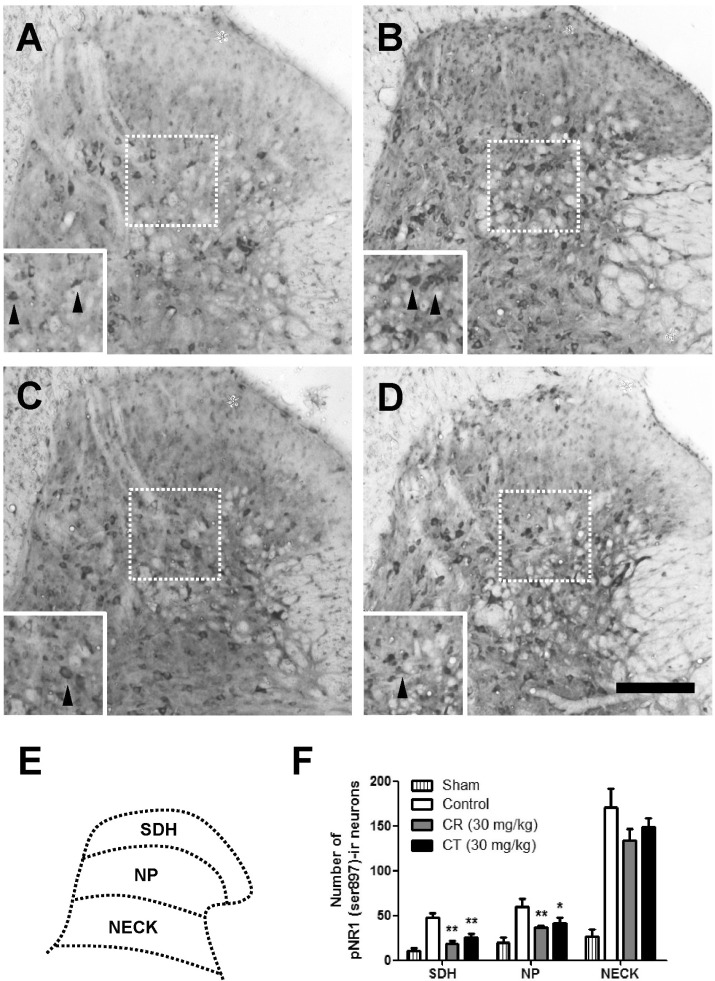
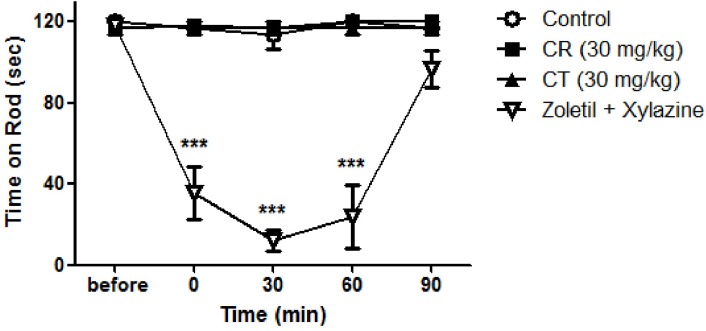
- In this study, we examined the antinociceptive effect of Cyperi rhizoma (CR) and Corydalis tuber (CT) extracts using a chronic constriction injury-induced neuropathic pain rat model. After the ligation of sciatic nerve, neuropathic pain behavior such as mechanical allodynia and thermal hyperalgesia were rapidly induced and maintained for 1 month. Repeated treatment of CR or CT (per oral, 10 or 30 mg/kg, twice a day) was performed either in induction (day 0~5) or maintenance (day 14~19) period of neuropathic pain state. Treatment of CR or CT at doses of 30 mg/kg in the induction and maintenance periods significantly decreased the nerve injury-induced mechanical allodynia. In addition, CR and CT at doses of 10 or 30 mg/kg alleviated thermal heat hyperalgesia when they were treated in the maintenance period. Finally, CR or CT (30 mg/kg) treated during the induction period remarkably reduced the nerve injury-induced phosphorylation of NMDA receptor NR1 subunit (pNR1) in the spinal dorsal horn. Results of this study suggest that extracts from CR and CT may be useful to alleviate neuropathic pain.
Keyword
MeSH Terms
Figure
Reference
-
1. Ding GS. Important Chinese herbal remedies. Clin Ther. 1987; 9:345–357. PMID: 3607815.2. Lee TH, Son M, Kim SY. Effects of corydaline from Corydalis tuber on gastric motor function in an animal model. Biol Pharm Bull. 2010; 33:958–962. PMID: 20522959.
Article3. Lee CH, Hwang DS, Kim HG, Oh H, Park H, Cho JH, Lee JM, Jang JB, Lee KS, Oh MS. Protective effect of Cyperi rhizoma against 6-hydroxydopamine-induced neuronal damage. J Med Food. 2010; 13:564–571. PMID: 20521982.
Article4. Bae H, Park N, Kim Y, Hong M, Shin M, Kim SH, Kim J. The modulative effect of Cyperi Rhizoma on Th1/Th2 lineage development. Cytokine. 2010; 51:259–265. PMID: 20580249.
Article5. Kim SH, Han J, Seog DH, Chung JY, Kim N, Hong Park Y, Lee SK. Antidepressant effect of Chaihu-Shugan-San extract and its constituents in rat models of depression. Life Sci. 2005; 76:1297–1306. PMID: 15642599.
Article6. Cheong BS, Choi DY, Cho NH, Lee JD, Chang HK, Shin MC, Shin MS, Kim CJ. Modulation of Corydalis tuber on glycine-induced ion current in acutely dissociated rat periaqueductal gray neurons. Biol Pharm Bull. 2004; 27:1207–1211. PMID: 15305023.
Article7. Kubo M, Matsuda H, Tokuoka K, Ma S, Shiomoto H. Anti-inflammatory activities of methanolic extract and alkaloidal components from Corydalis tuber. Biol Pharm Bull. 1994; 17:262–265. PMID: 7515744.8. Weenen H, Nkunya MH, Bray DH, Mwasumbi LB, Kinabo LS, Kilimali VA, Wijnberg JB. Antimalarial compounds containing an alpha,beta-unsaturated carbonyl moiety from Tanzanian medicinal plants. Planta Med. 1990; 56:371–373. PMID: 2236290.9. Weenen H, Nkunya MH, Bray DH, Mwasumbi LB, Kinabo LS, Kilimali VA. Antimalarial activity of Tanzanian medicinal plants. Planta Med. 1990; 56:368–370. PMID: 2236289.10. Ma WG, Fukushi Y, Tahara S, Osawa T. Fungitoxic alkaloids from Hokkaido Papaveraceae. Fitoterapia. 2000; 71:527–534. PMID: 11449501.
Article11. Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002; 5(Suppl):1062–1067. PMID: 12403987.
Article12. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999; 353:1959–1964. PMID: 10371588.
Article13. Rice AS, Hill RG. New treatments for neuropathic pain. Annu Rev Med. 2006; 57:535–551. PMID: 16409165.
Article14. Son JS, Kwon YB. Sigma-1 receptor antagonist BD1047 reduces allodynia and spinal ERK phosphorylation following chronic compression of dorsal root ganglion in rats. Korean J Physiol Pharmacol. 2010; 14:359–364. PMID: 21311675.
Article15. Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001; 429:23–37. PMID: 11698024.
Article16. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009; 139:267–284. PMID: 19837031.
Article17. Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998; 54:581–618. PMID: 9550192.
Article18. Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006; 399:85–90. PMID: 16469445.
Article19. Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005; 116:62–72. PMID: 15936881.
Article20. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988; 33:87–107. PMID: 2837713.
Article21. Kawasaki-Yatsugi S, Nagakura Y, Ogino S, Sekizawa T, Kiso T, Takahashi M, Ishikawa G, Ito H, Shimizu Y. Automated measurement of spontaneous pain-associated limb movement and drug efficacy evaluation in a rat model of neuropathic pain. Eur J Pain. 2012; 16:1426–1436. PMID: 22451419.
Article22. Kumar N, Laferriere A, Yu JS, Leavitt A, Coderre TJ. Evidence that pregabalin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2010; 113:552–561. PMID: 20132471.
Article23. Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, Beitz AJ, Lee JH. Depletion of capsaicin-sensitive afferents prevents lamina-dependent increases in spinal N-methyl-D-aspartate receptor subunit 1 expression and phosphorylation associated with thermal hyperalgesia in neuropathic rats. Eur J Pain. 2008; 12:552–563. PMID: 17933570.24. Roh DH, Kwon YB, Kim HW, Ham TW, Yoon SY, Kang SY, Han HJ, Lee HJ, Beitz AJ, Lee JH. Acupoint stimulation with diluted bee venom (apipuncture) alleviates thermal hyperalgesia in a rodent neuropathic pain model: involvement of spinal alpha 2-adrenoceptors. J Pain. 2004; 5:297–303. PMID: 15336634.25. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988; 32:77–88. PMID: 3340425.
Article26. Kwon YB, Jeong YC, Kwon JK, Son JS, Kim KW. The Antinociceptive Effect of Sigma-1 Receptor Antagonist, BD1047, in a Capsaicin Induced Headache Model in Rats. Korean J Physiol Pharmacol. 2009; 13:425–429. PMID: 20054487.
Article27. Abbadie C, Besson JM. Chronic treatments with aspirin or acetaminophen reduce both the development of polyarthritis and Fos-like immunoreactivity in rat lumbar spinal cord. Pain. 1994; 57:45–54. PMID: 8065795.
Article28. Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim). 1957; 46:208–209. PMID: 13502156.29. Vranken JH. Mechanisms and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem. 2009; 9:71–78. PMID: 20021340.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociceptive Effects of Amikacin on Neuropathic Pain in Rats
- Antinociceptive Effects of Tramadol on the Neuropathic Pain in Rats
- Regulation of glutamate level in rat brain through activation of glutamate dehydrogenase by Corydalis ternata
- DA-9701: A New Multi-Acting Drug for the Treatment of Functional Dyspepsia
- The Spermatogenic Effect of Yacon Extract and Its Constituents and Their Inhibition Effect of Testosterone Metabolism