Korean J Physiol Pharmacol.
2011 Apr;15(2):67-81. 10.4196/kjpp.2011.15.2.67.
Modern Methods for Analysis of Antiepileptic Drugs in the Biological Fluids for Pharmacokinetics, Bioequivalence and Therapeutic Drug Monitoring
- Affiliations
-
- 1Pharmacology & Clinical Pharmacology Lab, College of Medicine, Hanyang University, Seoul 133-791, Korea. jskang@hanyang.ac.kr
- 2Global Medical Science, College of Nursing, Sungshin Women's University, Seoul 142-100, Korea.
- KMID: 2285410
- DOI: http://doi.org/10.4196/kjpp.2011.15.2.67
Abstract
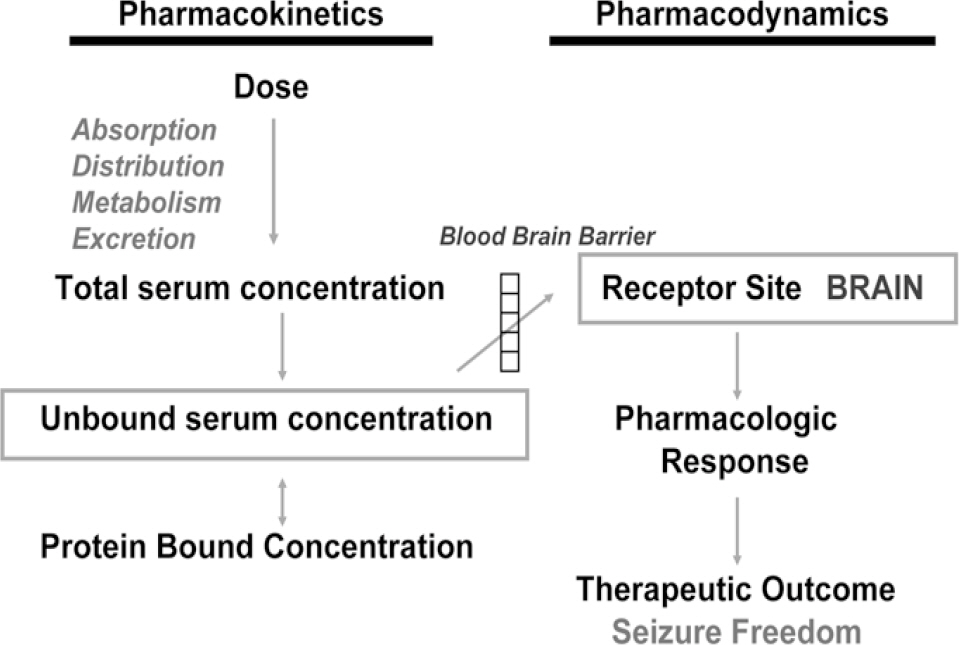
- Epilepsy is a chronic disease occurring in approximately 1.0% of the world's population. About 30% of the epileptic patients treated with availably antiepileptic drugs (AEDs) continue to have seizures and are considered therapy-resistant or refractory patients. The ultimate goal for the use of AEDs is complete cessation of seizures without side effects. Because of a narrow therapeutic index of AEDs, a complete understanding of its clinical pharmacokinetics is essential for understanding of the pharmacodynamics of these drugs. These drug concentrations in biological fluids serve as surrogate markers and can be used to guide or target drug dosing. Because early studies demonstrated clinical and/or electroencephalographic correlations with serum concentrations of several AEDs, It has been almost 50 years since clinicians started using plasma concentrations of AEDs to optimize pharmacotherapy in patients with epilepsy. Therefore, validated analytical method for concentrations of AEDs in biological fluids is a necessity in order to explore pharmacokinetics, bioequivalence and TDM in various clinical situations. There are hundreds of published articles on the analysis of specific AEDs by a wide variety of analytical methods in biological samples have appears over the past decade. This review intends to provide an updated, concise overview on the modern method development for monitoring AEDs for pharmacokinetic studies, bioequivalence and therapeutic drug monitoring.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Anderson GD. Pharmacokinetic, Pharmacodynamic, and pharmacogenetic targeted therapy of antiepileptic drugs. Ther Drug Monit. 2008; 30:173–180.
Article2. Chollet DF. Determination of antiepileptic drugs in biological material. J Chromatogr B Analyt Technol Biomed Life Sci. 2002; 767:191–233.
Article3. Johannessen SI. Plasma drug concentration monitoring of anticonvulsants: practical guidelines. CNS Drugs. 1997; 7:349–365.4. Eadie MJ. Therapeutic drug monitoring-antiepileptic drugs. Br J Clin Pharmacol. 1998; 46:185–193.5. Buchthal F, Svensmark O. Aspects of pharmacology of phenytoin (dilantin) and phenobarbital relevant to their dosage in the treatment of epilepsy. Epilepsia. 1960; 1:373–384.6. Kutt H, Penry JK. Usefulness of blood levels of antiepileptic drugs. Arch Neurol. 1974; 31:283–288.
Article7. Johannessen SI, Battino D, Berry DJ, Bialer M, Kramer G, Tomson T, Patsalos PN. Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit. 2003; 25:347–363.
Article8. Patsalos PN. New antiepileptic drugs. Ann Clin Biochem. 1999; 36:10–19.
Article9. Glauser TA, Pippenger CE. Controversies in blood-level monitoring; reexamining its role in the treatment of epilepsy. Epilepsia. 2000; 41 Suppl. 8:S6–15.
Article10. Solden SJ, Hill JG. Rapid micromethod for measuring anticonvulsant drugs in serum by high-performance liquid chromatography. Clin Chem. 1976; 22:856–859.11. Kabra PM, Stafford BE, Marton LJ. Simultaneous measurement of phenobarbital, phenytoin, primidone, ethosuximide, and carbamazepine in serum by high-performance liquid chromatography. Clin Chem. 1977; 23:1284–1288.12. Christofides JA, Fry DE. Measurement of anticonvulsants in serum by reversed-phase ion-pair liquid chromatography. Clin Chem. 1980; 26:499–501.
Article13. Ou CN, Robnerud CL. Simultaneous measurement of ethosuximide, primidone, phenobarbital, phenytoin, carbamazepine, and their bioactive metabolites by liquid chromatography. Clin Chem. 1984; 30:1667–1670.
Article14. Juergens U. Simultaneous determination of zonisamide and none other antiepileptic drugs and metabolites in serum. A comparison of microbore and conventional high-performance liquid chromatography. J Chromatogr. 1987; 385:233–240.15. Lanças FM, Sozza MA, Queiroz ME. Simultaneous plasma lamotrigine analysis with carbamazepine, carbamazepine-10,11-epoxide, primidone, phenytoin, phenobarbital, and PEMA by micellar electrokinetic capillary chromatography (MECC). J Anal Toxicol. 2003; 27:304–308.16. Romanyshyn LA, Wichmann JK, Kucharczyk N, Shumaker RC, Ward D, Sofia RD. Simultaneous determination of felbamate, primidone, Phenobarbital, carbamazepine, two carbamazepine metabolites, phenytoin and one phenytoin metabolite in human plasma by high-performance liquid chromatography. Ther Drug Monit. 1994; 16:90–99.
Article17. Kataoka Y, Makino K, Oishi R. Capillary electrophoresis for therapeutic drug monitoring of antiepileptics. Electrophoresis. 1998; 19:2856–2860.
Article18. Burton ME, Shaw LM, Schentag JJ. Antiepileptic drugs. Garnett WR, Anderson GD, Collins RJ, editors. Applied pharmacokinetics & pharmacodynamics – principles of therapeutic drug monitoring. 4th ed.Philadelphia: Lippincott Williams & Wilkins;2006. p. 491.19. Graves NM, Garnett WR. Epilepsy. Dipiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: A Pathophysiologic Approach. 6th ed.New York: McGraw-Hill;2005. p. 1023.20. USP Drug Information. Volume I: Drug Information for the Health Care Professional. 24th ed.Bethesda: Greenwood Village Co;2004. p. 709.21. Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC, Glauser TA, Johannessen SI, Leppik IE, Tomson T, Perucca E. Antiepileptic drugs-best practice guidelines for therapeutic drug monitoring: a position paper by the subcommision on therapeutic drug monitoring, ILAE commission on therapeutic strategies. Epilepsia. 2008; 49:1239–1276.22. Rambeck B, May TW, Jürgens MU, Blankenhorn V, Jürges U, Korn-Merker E, Sälke-Kellermann A. Comparison of phenytoin and carbamazepine serum concentrations measured by high-performance liquid chromatography, the standard TDx assay, the enzyme multiplied immunoassay technique, and a new patient-side immunoassay cartridge system. Ther Drug Monit. 1994; 16:608–612.
Article23. Sánchez A, Garcia R, Abadin JA, Durán JA. Determination of free serum carbamazepine by protein precipitation with sulphosalicylic acid. Pharm Pharmacol Commun. 1999; 5:435–438.
Article24. Bhatti MM, Hanson GD, Schultz L. Simultaneous determination of phenytoin, carbamazepine, and 10,11-carbamazepine epoxide in human serum by high-performance liquid chromatography with ultra-violet detection. J Pharm Biomed Anal. 1998; 16:1233–1240.25. Queiroz ME, Silva SM, Carvalho D, Lancas FM. Determination of lamotrigine simultaneously with carbamazepine, carbamazepine epoxide, phenytoin, Phenobarbital, and primidone in human plasma by SPME-GC-TSD. J Chromatogr Sci. 2002; 40:219–223.
Article26. Yoshida T, Imai K, Motohashi S, Hamano S, Sato M. Simultaneous determination of zonisamide, carbamazepine and carbamazepine-10,11-epoxide in infant serum by high-performance liquid chromatography. J Pharm Biomed Anal. 2006; 41:1386–1390.
Article27. Oh E, Ban E, Woo JS, Kim CK. Analysis of carbamazepine and its active metabolite, carbamazepine-10,11-epoxide, in human plasma using high-performance liquid chromatography. Anal Bioanal Chem. 2006; 386:1931–1936.
Article28. Leite CE, Petersen GO, Lunardelli A, Thiesen FV. A high-performance liquid chromatography method for the determination of carbamazepine and carbamazepine-10,11-epoxide and its comparison with chemiluminescent immunoassay. Clin Chem Lab Med. 2009; 47:458–463.
Article29. Breton H, Cociglio M, Bressolle F, Peyriere H, Blayac JP, Hillaire-Buys D. Liquid chromatography-electrospray mass spectrometry determination of carbamazepine, oxcarbazepine and eight of their metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 828:80–90.
Article30. Subramanian M, Birnbaum AK, Remmel RP. High-speed simultaneous determination of nine antiepileptic drugs using liquid chromatography-mass spectrometry. Ther Drug Monit. 2008; 30:347–356.
Article31. Vuckovic D, de Lannoy I, Gien B, Yang Y, Musteata FM, Shirey R, Sidisky L, Pawliszyn J. In vivo solid-phase microextration for single rodent pharmacokinetics studies of carbamazepine and carbamazepine-10, 11-epoxide in mice. J Chromatogr A. 2010; 60:1060–1068.32. Aucamp AK. Aspects of the pharmacokinetics and pharmcodynamics of benzodiazepines with particular reference to clobazam. Drug Dev Res Suppl. 1982; 1:117–126.33. Contin M, Sangiorgi S, Riva R, Parmeggiani A, Albani F, Baruzzi A. Evidence of polymorphism CYP2C19 involvement in the human metabolism of N-desmethylclobazam. Ther Drug Monit. 2002; 24:737–741.34. Aylett SE, Cross JH, Berry D. Clobazam toxicity in a child with epilepsy related to idiosyncratic metabolism. Dev Med Child Neurol. 2005; 48:612–615.35. LeGatt DF, McIntosh DP. Clobazam and norclobazam quantitation in serum by capillary gas chromatography with electron-capture detection. Clin Biochem. 1993; 26:159–163.
Article36. Gaillard Y, Gay-Montchamp JP, Ollagnier M. Simultaneous screening and quantitation of alpidem, zolpidem, buspirone and benzodiazepines by dual-channel gas chromatography using electron-capture and nitrogen-phosphorus detection after solid-phase extraction. J Chromatogr. 1993; 622:197–208.
Article37. Maurer H, Pfleger K. Identification and differentiation of benzodiazepines and their metabolites in urine by computerized gas chromatography-mass spectrometry. J Chromatogr. 1987; 422:85–101.
Article38. Drouet-Coassolo C, Aubert C, Coassolo P, Cano JP. Capillary gas chromatographic-mass spectrometric method for the identification and quantification of some benzodiazepines and their unconjugated metabolites in plasma. J Chromatogr. 1989; 487:295–311.
Article39. Akerman KK. Analysis of clobazam and its active metabolite norclobazam in plasma and serum using HPLC/DAD. Scand J Clin Lab Invest. 1996; 56:609–614.40. Knapp J, Boknik P, Gumbinger HG, Linck B, Lüss H, Müller FU, Schmitz W, Vahlensieck U, Neumann J. Quantitation of clobazam in human plasma using high-performance liquid chromatography. J Chromatogr Sci. 1999; 37:145–149.
Article41. Kunicki PK. Simple and sensitive high-performance liquid chromatographic method for the determination of 1,5-benzodiazepine clobazam and its active metabolite N-desmethylclobazam in human serum and urine with application to 1,4-benzodiazepines analysis. J Chromatogr B Biomed Sci Appl. 2001; 750:41–49.
Article42. Irving RC, Dickson SJ. The detection of sedatives in hair and nail samples using tandem LC-MS-MS. Forensic Sci Int. 2007; 166:58–67.
Article43. Nakamura M, Ohmori T, Itoh Y, Terashita M, Hirano K. Simultaneous determination of benzodiazepines and their metabolites in human serum by liquid chromatographytandem mass spectrometry using a high-resolution octadecyl silica column compatible with aqueous compounds. Biomed Chromatogr. 2009; 23:357–364.
Article44. Dreifuss FE, Penry JK, Rose SW, Kupferberg HJ, Dyken P, Sato S. Serum clonazepam concentrations in children with absence seizures. Neurology. 1975; 23:255–258.
Article45. Hvidberg EF, Dam M. Clinical pharmacokinetics of anticonvulsants. Clin Pharmacokinet. 1976; 1:161–188.
Article46. Sjö O, Hvidberg EF, Naestoft J, Lund M. Pharmacokinetics and side-effects of clonazepam and its 7-amino-metabolite in man. Eur J Clin Pharmacol. 1975; 8:249–254.47. Berlin A, Dahlström H. Pharmacokinetics of the anticonvulsant drug clonazepam evaluated from single oral and intravenous doses and by repeated oral administration. Eur J Clin Pharmacol. 1975; 9:155–159.
Article48. Labbate LA, Pollack MH, Otto MW, Tesar GM, Rosenbaum JF. The relationship of alprazolam and clonazepam dose to steady-state concentration in plasma. J Clin Psychopharmacol. 1994; 14:274–276.
Article49. Yukawa E, Satou M, Neonaka T, Yukawa M, Ohdo S, Higuchi S, Kuroda T, Goto Y. Influence of age and comedication on steady-state clonazepam serum level-dose ratios in Japanese epileptic patients. J Clin Pharm Ther. 2001; 26:375–379.
Article50. de Carvalho D, Lanchote VL. Measurement of plasma clonazepam for therapeutic control: a comparison of chromatographic methods. Ther Drug Monit. 1991; 13:55–63.51. Dhar AK, Kutt H. Improved gas chromatographic procedure for the determination of clonazepam levels in plasma using a nitrogen-sensitive detector. J Chromatogr. 1981; 222:203–211.
Article52. Tiscione NB, Shan X, Alford I, Yeatman DT. Quantitation of benzodiazepines in whole blood by electron impact-gas chromatography-mass spectrometry. J Anal Toxicol. 2008; 32:644–652.
Article53. Gunnar T, Ariniemi K, Lillsunde P. Fast gas chromatography-negative-ion chemical ionization mass spectrometry with microscale volume sample preparation for the determination of benzodiazepines and alpha-hydroxy metabolites, zaleplon and zopiclone in whole blood. J Mass Spectrom. 2006; 41:741–754.54. El Mahjoub A, Staub C. High-performance liquid chromatographic method for the determination of benzodiazepines in plasma or serum using the column-switching technique. J Chromatogr B Biomed Sci Appl. 2000; 742:381–390.
Article55. Tanaka E, Terada M, Misawa S, Wakasugi C. Simultaneous determination of twelve benzodiazepines in human serum using a new reversed-phase chromatographic column on a 2-microns porous microspherical silica gel. J Chromatogr B Biomed Appl. 1996; 682:173–178.56. Le Quellec C, Gaudet ML, Breteau M. Improved selectivity for high-performance liquid chromatographic determination of clonazepam in plasma of epileptic patients. J Chromatogr B Biomed Sci Appl. 1998; 719:227–233.57. Salem AA, Barsoum BN, Izake EL. Spectrophotometric and fluorimetric determination of diazepam, bromazepam and clonazepam in pharmaceutical and urine samples. Spectrochim Acta A Mol Biomol Spectrosc. 2004; 60:771–780.
Article58. Cavedal LE, Mendes FD, Dominques CC, Patni AK, Monif T, Reyar S, Pereira Ados S, Mendes GD, De Nucci G. Clonazepam quantification in human plasma by high-performance liquid chromatography coupled with electrospray tandem mass spectrometry in a bioequivalence study. J Mass Spectrom. 2007; 42:81–88.
Article59. Agarwal S, Gowda KV, Selvan PS, Chattaraj TK, Pal TK. Bioequivalence of two commercial preparations of escitalopram oxalate/clonazepam using a liquid chromatography-electrospray mass spectrometry method. Arzeimittelforschung. 2008; 58:551–556.
Article60. Marin SJ, Coles R, Merrell M, McMillin GA. Quantitation of benzodiazepines in urine, serum, plasma, and meconium by LC-MS-MS. J Anal Toxicol. 2008; 32:491–498.
Article61. Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989; 25:582–593.
Article62. Brodies MJ, Dichter MA. Antiepileptic drugs. N Eng J Med. 1996; 334:168–175.63. Neels HM, Sierens AC, Naelaerts K, Scharpė SL, Hatfield GM, Lambert WE. Therapeutic drug monitoring of old and newer anti-epileptic drugs. Clin Chem Lab Med. 2004; 42:1228–1255.
Article64. Miles MV, Howlett CM, Tennison MB, Greenwood RS, Cross RE. Determination of N-desmethylmethsuximide serum concentrations using enzyme-multiplied and fluorescence polarization immunoassays. Ther Drug Monit. 1989; 11:337–342.
Article65. Matar KM, Nicolls PJ, Tekle A, Bawazir SA, Al-Hassan MI. Liquid chromatographic determination of six antiepileptic drugs and two metabolites in microsamples of human plasma. Ther Drug Monit. 1999; 21:559–566.
Article66. Chen SH, Wu HL, Shen MC, Kou HS. Trace analysis of ethosuximide in human plasma with a chemically removable derivatizing reagent and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999; 729:111–117.
Article67. Sghendo L, Mifsud J, Ellul-Micallef R, Portelli J, Millership JS. A sensitive gas chromatographic/mass spectrometric method for the resolution and quantification of ethosuximide enantiomers in biological fluids. J Chromatogr B Analyt Technol Biomed Life Sci. 2002; 772:307–315.
Article68. Cacas MN, Blanco CC, Carretero AS, Gutiėrrez AF. Simple and rapid micellar electrokinetic capillary chromatographic method for simultanoeus determination of four antiepileptics in human serum. Biomed Chromatogr. 2004; 18:608–612.69. Bhatt M, Shash S, Shivprakash . Development of a high-throughput method for the determination of ethosuximide in human plasma by liquid chromatography mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:1605–1610.
Article70. Johannessen SI, Tomson T. Laboratory monitoring of antiepileptic drugs. Levy RH, Mattson RH, Meldrum BS, editors. Methods of determination of antiepileptic drugs in anti-epileptic drugs. 4th ed.Philadelphia: LWW;2002. p. 103.71. Ermakov AN, Bohrina DE, Egorov AM, Eremin SA. Polarized fluoroimmunoanalysis of phenobarbital using the TD(x) analyzer from “Abbott” laboratories. Vopr Med Khim. 1993; 39:59–63.72. Dzegoev AB, Eremin SA, Karpov MV, Danilova NP, Vasilova RG, Egorov AM. Determination of phenobarbital by polarized fluoroimmunoanalysis. The effect of immunogen structure and tracer on specificity and detection limit. Bioorg Khim. 1993; 19:713–721.73. Maresova V, Chadt J, Novakova E. Screening and semiquantitative analysis of drugs and drugs of abuse in human serum samples using gas chromatography-mass spectrometry. Neuro Endocrinol Lett. 2008; 29:749–754.74. Saka K, Uemura K, Shintani-Ishida K, Yoshida K. Determination of amobarbital and phenobarbital in serum by gas chromatography-mass spectrometry with addition of formic acid to the solvent. J Chromatogr B Analyt Technol Biomed Life Sci. 2008; 869:9–15.
Article75. Zhao H, Wang L, Qiu Y, Zhou Z, Zhong W, Li X. Multiwalled carbon nanotubes as a solid-phase extraction adsorbent for the determination of three barbiturates in pork by ion trap gas chromatography-tandem mass spectrometry (GC/MS/MS) following microwave assisted derivatization. Anal Chim Acta. 2007; 586:399–406.
Article76. Chen K, Khayam-Bashi H. Comparative analysis of anti-epileptic drugs by gas chromatography using capillary or packed columns and by fluorescence polarization immunoassay. J Anal Toxicol. 1991; 15:82–85.
Article77. Patil KM, Bodhankar SL. Simultaneous determination of lamotrigine, phenobarbitone, carbamazepine and phenytoin in human serum by high-performance liquid chromatography. J Pharm Biomed Anal. 2005; 39:181–186.
Article78. Kishore P, Rajnarayana K, Reddy MS, Sagar JV, Krishna DR. Validated high performance liquid chromatographic method for simultaneous determination of phenytoin, phenobarbital and carbamazepine in human serum. Arzeimittelforschung. 2003; 53:763–768.
Article79. Queiroz RH, Bertucci C, Malfará WR, Dreossi SA, Chaves AR, Valėrio DA, Queiroz ME. Quantification of carbamazepine, carbamazepine-10,11-epoxide, phenytoin and phenobarbital in plasma samples by stir bar-sorptive extraction and liquid chromatography. J Pharm Biomed Anal. 2008; 48:428–834.
Article80. Heideloff C, Bunch DR, Wang S. A novel HPLC method for quantification of 10 antiepileptic drugs or mewtabolites in serum/plasma using a monolithic column. Ther Drug Monit. 2010; 32:102–106.81. DeLuccia FJ, Arunyanart M, Love LJ. Direct serum injection with micellar liquid chromatography for therapeutic drug monitoring. Anal Chem. 1985; 57:1564–1568.
Article82. Ceccato A, Boulanger B, Chiap P, Hubert P, Crommen J. Simultaneous determination of methylphenobarbital enantiomers and phenobarbital in human plasma by on-line coupling of an achiral precolumn to a chiral liquid chromatographic column. J Chromatogr A. 1998; 819:143–153.
Article83. Winter ME, Tozer TN. Phenytoin. Burton ME, Shaw LM, Schentag JJ, Evans WE, editors. Applied pharmacokinetics & Pharmacodynamic: principles of therapeutic drug monitoring. 4rd ed.Philadelphia: LWW;2006. p. 464.84. Tozer TN, Winter ME. Phenytoin. Evans WE, Schentag JJ, Jusko WJ, editors. Applied pharmacokinetics: principles of therapeutic drug monitoring. 3rd ed.Vancouver WA: Applied Therapeutics;1992. p. 25.85. Tutor-Crespo MJ, Hermida J, Tutor JC. Phenytoin immunoassay measurements in serum samples from patients with renal insufficiency: comparison with high-performance liquid chromatography. J Clin Lab Anal. 2007; 21:119–123.
Article86. Kugler AR, Annesley TM, Nordblom GD, Koup JR, Olson SC. Cross-reactivity of fosphenytoin in two human plasma phenytoin immunoassays. Clin Chem. 1998; 44:1474–1480.
Article87. Roberts WL, De BK, Coleman JP, Annesley TM. Falsely increased immunoassay measurements of total and unbound phenytoin in critically ill uremic patients receiving fosphenytoin. Clin Chem. 1999; 45:829–837.
Article88. Sirgo MA, Green PJ, Rocci ML Jr, Vlasses PH. Interpretation of serum phenytoin concentrations in uremia is assay-dependent. Neurology. 1984; 34:1250–1251.
Article89. Külpmann WR, Oellerich M. Monitoring of therapeutic serum concentrations of antiepileptic drugs by a newly developed gas chromatographic procedure and enzyme immunoassay (EMIT): a comparative study. J Clin Chem Clin Biochem. 1981; 19:249–258.
Article90. Zhang Y, Mehrotra N, Budha NR, Christensen ML, Meibohm B. A tandem mass spectrometry assay for the simultaneous determination of acetaminophen, caffeine, phenytoin, ranitidine, and theophylline in small volume pediatric plasma specimens. Clin Chim Acta. 2008; 398:105–112.
Article91. Levert H, Odou P, Robert H. Simultaneous determination of four antiepileptic drugs in serum by high-performance liquid chromatography. Biomed Chromatogr. 2002; 16:19–24.
Article92. Rezaei Z, Hemmateenejad B, Khabnadideh S, Gorgin M. Simultaneous spectrophotometric determination of carbamazepine and phenytoin in serum by PLS regression and comparison with HPLC. Talanta. 2005; 65:21–28.
Article93. Rating D, Nau H, Jäger-Roman E, Göpfert-Geyer I, Koch S, Beck-Mannagetta G, Schmidt D, Helge H. Teratogenic and pharmacokinetic studies of primidone during pregnancy and in the offspring of epileptic women. Acta Paediatr Scand. 1982; 71:301–311.
Article94. Booker HE, Hosokowa K, Burdette RR, Darcey B. A clinical study of serum primidone levels. Epilepsia. 1970; 11:395–402.
Article95. Carl GF, Smith DB, Bridges G. Comparison of fluorescent immunoassay and EMIT for assay of serum primidone concentration. Ther Drug Monit. 1982; 4:325–328.
Article96. Knight D. Enzyme immunoassay of phenobarbital, phenytoin, primidone, carbamazepine and theophylline with the Abbott VP Bichromatic Analyzer. Clin Biochem. 1981; 14:14–15.
Article97. Moriyama M, Furuno K, Oishi R, Gomita Y. Simultaneous determination of primidone and its active metabolites in rat plasma by high-performance liquid chromatography using a solid-phase extraction technique. J Pharm Sci. 1994; 83:1751–1753.
Article98. Budakova L, Brozmanova H, Grundmann M, Fischer J. Simultaneous determination of antiepileptic drugs and their two active metabolites by HPLC. J Sep Sci. 2008; 31:1–8.
Article99. Treston AM, Hooper WD. Urinary metabolites of phenobarbitone, primidone, and their N-methyl and N-ethyl derivatives in humans. Xenobiotica. 1992; 22:385–394.100. Maurer HH. Detection of anticonvulsants and their metabolites in urine within a “general unknown” analysis procedure using computerized gas chromatography-mass spectrometry. Arch Toxicol. 1990; 64:554–561.
Article101. Drummer OH. Chromatographic screening techniques in systematic toxicological analysis. J Chromatogr B Biomed Sci Appl. 1999; 733:27–45.
Article102. Marquet P. Is LC-MS suitable for a comprehensive screening of drugs and poisons in clinical toxicology. Ther Drug Monit. 2002; 24:125–133.
Article103. Wilder BJ, Rangel RJ. Review of valproate monotherapy in the treatment of generalized tonic-clonic seizures. Am J Med. 1988; 84:7–13.
Article104. Baillie TA. Metabolism of valproate to hepatotoxic intermediates. Pharm Weekbl Sci. 1992; 14:122–125.
Article105. Tisdale JE, Tsuyuki RT, Oles KS, Penry JK. Relationship between serum concentration and dose of vaproic acid during monotherapy in adult outpatients. Ther Drug Monit. 1992; 14:416–423.106. Gidal BE, Pitterle ME, Spencer NW, Maly MM. Relationship between vaproic acid dosage, plasma concentration and clearance in adult monotherapy patients with epilepsy. J Clin Pharm Ther. 1995; 20:215–219.107. Cloyd JC, Fischer JH, Kriel RL, Kraus DM. Vaproic acid pharmacokinetics in children. IV. Effects of age and anti-epileptic drugs on protein binding and intrinsic clearance. Clin Pharmacol Ther. 1993; 53:22–29.108. Fattore C, Messina S, Battino D, Croci D, Mamoli D, Perucca E. The influence of old age and enzyme inducing comedication on the pharmacokinetics of vaproic acid at steady-state: a case-matched evaluation based on therapeutic drug monitoring data. Epilepsy Res. 2006; 70:153–160.109. Perucca E, Aldenkamp A, Tallis R, Krämer G. Role of vaproate across the ages. Treatment of epilepsy in the elderly. Acta Neurol Scand Suppl. 2006; 184:28–37.110. Darius J, Meyer FP. Sensitive capillary gas chromatographic-mass spectrometric method for the therapeutic drug monitoring of vaproic acid and seven of its metabolites in human serum. Application of the assay for a group of pediatric epileptics. J Chromatogr B Biomed Appl. 1994; 656:343–351.111. Yu D, Gordon JD, Zheng J, Panesar SK, Riggs KW, Rurak DW, Abbott FS. Determination of vaproic acid and its metabolites using gas chromatography with mass-selective detection: application to serum and urine samples from sheep. J Chromatogr B Biomed Appl. 1995; 666:269–281.112. Sugimoto T, Muro H, Woo M, Nishida N, Murakami K. Valproate metabolites in high-dose valproate plus phenytoin therapy. Epilepsia. 1996; 37:1200–1203.
Article113. Yang X, Janatova J, Juenke JM, McMillin GA, Andrade JD. An ImmunoChip prototype for simultaneous detection of antiepileptic drugs using an enhanced one-step homogeneous immunoassay. Anal Biochem. 2007; 365:222–229.
Article114. Steijns LS, Bouw J, van der Weide J. Evaluation of fluorescence polarization assays for measuring vaproic acid, phenytoin, carbamazepine and phenobarbital in serum. Ther Drug Monit. 2002; 24:432–435.115. Xiao H, Zhang S. Determination of valproic acid level in serum by high performance liquid chromatography. Se Pu. 1998; 16:365–366.116. Amini H, Javan M, Ahmadiani A. Development and validation of a sensitive assay of vaproic acid in human plasma by high-performance liquid chromatography without prior derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2006; 830:368–371.117. Zhong Y, Jiao Z, Yu Y. Simultaneous determination of mycophenolic acid and valproic acid based on derivatization by high-performance liquid chromatography with fluorescence detection. Biomed Chromatogr. 2006; 20:319–326.
Article118. Lin MC, Kou HS, Chen CC, Wu SM, Wu HL. Simple and sensitive fluorimetric liquid chromatography method for the determination of valproic acid in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004; 810:169–172.
Article119. Kishore P, Rajani Kumar V, Satyanarayana V, Krishna DR. HPLC determination of valproic acid in human serum. Pharmazie. 2003; 58:378–380.120. Allain P, Turcant A, Prémel-Cabic A. Automated fluoroimmunoassay of theophylline and valproic acid by flow-injection analysis with use of HPLC instruments. Clin Chem. 1989; 35:469–470.
Article121. Tosoni S, Signorini C, Albertini A. Evaluation of valproic acid fluoroimmunoassay and comparison with a gas liquid chromatographic method. Ther Drug Monit. 1985; 7:236–238.
Article122. Liu H, Montoya JL, Forman LJ, Eggers CM, Barham CF, Delgado M. Determination of free valproic acid: evaluation of the Centrifree system and comparison between high-performance liquid chromatography and enzyme immunoassay. Ther Drug Monit. 1992; 4:513–521.123. Liu H, Forman LJ, Montoya J, Eggers C, Barham CF, Delgado M. Determination of valproic acid by high-performance liquid chromatography with photodiode-array and fluorescence detection. J Chromatogr. 1992; 576:163–169.
Article124. Deng C, Li N, Ji J, Yang B, Duan G, Zhang X. Development of water-phase derivatization followed by solid-phase micro-extraction and gas chromatography/mass spectrometry for fast determination of valproic acid in human plasma. Rapid Commun Mass Spectrom. 2006; 20:1281–1287.
Article125. Cheng H, Liu Z, Blum W, Byrd JC, Klisovic R, Grever MR, Marcucci G, Chan KK. Quantification of vaproic acid and its metabolite 2-propyl-4-pentenoic acid in human plasma using HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 850:206–212.126. Rho JM, Donevan SD, Rogawski MA. Mechanism of action of the anticonvulsant felbamate: opposing effects on N-methyl-D-aspartate and gamma-aminobutyric acidA receptors. Ann Neurol. 1994; 35:229–234.127. Palmer KJ, McTavish D. Felbamate: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in epilepsy. Drugs. 1993; 45:1041–1065.128. Pellock JM. Felbamate in epilepsy therapy; evaluating the risks. Drug Saf. 1999; 3:225–239.129. Gur P, Poklis A, Saady J, Costantino A. Chromatographic procedures for the determination of felbamate in serum. J Anal Toxicol. 1995; 19:499–503.
Article130. Poquette MA. Isothermal gas chromatographic method for the rapid determination of felbamate concentration in human serum. Ther Drug Monit. 1995; 17:168–173.
Article131. Rifai N, Fuller D, Law T, Mikati M. Measurement of felbamate by wide-bore capillary gas chromatography and flame ionization detection. Clin Chem. 1994; 40:745–748.
Article132. Tang PH. Drug monitoring and toxicology: a simple procedure for the monitoring of felbamate by HPLC-UV detection. J Anal Toxicol. 2008; 32:373–378.
Article133. Paw B, Misztal G, Skibiński R. Rapid and simple high-performance liquid chromatographic determination of felbamate in serum. Acta Pol Pharm. 2003; 60:339–342.134. Contin M, Balboni M, Calleqati E, Candela C, Albani F, Riva R, Baruzzi A. Simultaneous liquid chromatographic determination of lamotrigine, oxcarbazepine monohydroxy derivative and felbamate in plasma of patients with epilepsy. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 828:113–117.
Article135. Hansen RJ, Samber BJ, Gustafson DL. Rapid and sensitive LC-MS/MS method for determination of felbamate in mouse plasma and tissues and human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:3432–3436.
Article136. Shihabi ZK, Oles KS. Felbamate measured in serum by two methods: HPLC and capillary electrophoresis. Clin Chem. 1994; 40:1904–1908.
Article137. Thompson CD, Barthen MT, Hopper DW, Miller TA, Quigg M, Hudspeth C, Montouris G, Marsh L, Perhach JL, Sofia RD, Macdonald TL. Quantification in patient urine samples of felbamate and three metabolites: acid carbamate and two mercapturic acids. Epilepsia. 1999; 40:769–776.
Article138. Roller SG, Dieckhaus CM, Santos WL, Sofia RD, Macdonald TL. Interaction between human serum albumin and the felbamate metabolites 4-Hydroxy-5-phenyl-[1,3]oxazinan-2-one and 2-phenylpropenal. Chem Res Toxicol. 2002; 15:815–824.
Article139. McLean MJ. Clinical pharmacokinetics of gabapentin. Neurology. 1994; 44:17–22.140. Gidal BE, DeCerce J, Bockbrader HN, Gonzales Z, Kruger S, Pitterle ME, Rutccki P, Ramsay RE. Gabapentin bioavailability: effect of dose and frequency of administration in adult patients with epilepsy. Epilepsy Res. 1998; 31:91–99.
Article141. Stewart BH, Kugler AR, Thompson PR, Bockbrader HN. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res. 1993; 10:276–281.142. Berry DJ, Beran RG, Plunkeft MJ, Clarke LA, Hung WT. The absorption of gabapentin following high dose escalation. Seizure. 2003; 12:28–36.
Article143. Tomson T, Johannessen SI. Therapeutic monitoring of the new antiepileptic drugs. Eur J Clin Pharmacol. 2000; 55:697–705.
Article144. Armijo JA, Pena MA, Adín J, Vega-Gil N. Association between patient age and gabapentin serum concentration-to-dose ratio: a preliminary multivariate analysis. Ther Drug Monit. 2004; 26:633–637.145. Gidal BE, Radulovic LL, Kruger S, Rutecki P, Pitterle M, Bockbrader HN. Inter- and intra-subject variability in gabapentin absorption and absolute bioavailability. Epilepsy Res. 2000; 40:123–127.
Article146. Jalalizadeh H, Souri E, Tehrani MB, Jahangiri A. Validated HPLC method for the determination of gabapentin in human plasma using pre-column derivatization with 1-fluoro-2,4-dinitrobenzene and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 854:43–47.
Article147. Sagirli O, Cetin SM, Onal A. Determination of gabapentin in human plasma and urine by high-performance liquid chromatography with UV-vis detection. J Pharm Biomed Anal. 2006; 42:618–624.
Article148. Bahrami G, Kiani A. Sensitive high-performance liquid chromatographic quantitation of gabapentin in human serum using liquid-liquid extraction and pre-column derivatization with 9-fluorenylmethyl chloroformate. J Chromatogr B Analyt Technol Biomed Life Sci. 2006; 835:123–126.
Article149. Wolf CE, Saady JJ, Poklis A. Determination of gabapentin in serum using solid-phase extraction and gas-liquid chromatography. J Anal Toxicol. 1996; 20:498–501.
Article150. Ifa DR, Falci M, Moraes ME, Bezerra FA, Moraes MO, de Nucci G. Gabapentin quantification in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry. Application to bioequivalence study. J Mass Spectrom. 2001; 36:188–194.
Article151. Gambelunghe C, Mariucci G, Tantucci M, Ambrosini MV. Gas chromatography-tandem mass spectrometry analysis of gabapentin in serum. Biomed Chromatogr. 2005; 19:63–67.
Article152. Park JH, Jhee OH, Park SH, Lee JS, Shaw LM, Kim KH, Lee JH, Kim YS, Kang JS. Validated LC-MS/MS method for quantification of gabapentin in human plasma: application to pharmacokinetic and bioequivalence studies in Korean volunteers. Biomed Chromatogr. 2007; 21:829–835.
Article153. Ji HY, Jeong DW, Kim YH, Kim HH, Yoon YS, Lee KC, Lee HS. Determination of gabapentin in human plasma using hydrophilic interaction liquid chromatography with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006; 20:2127–2132.
Article154. Morris RG, Black AB, Harris AL, Batty AB, Sallustio BC. Lamotrigine and therapeutic drug monitoring: retrospective survey following the introduction of a routine service. Br J Clin Pharmacol. 1998; 46:547–551.
Article155. Fitton A, Goa KL. Lamotrigine. An update of its pharmacology and therapeutic use in epilepsy. Drugs. 1995; 50:691–713.156. Hirsch LJ, Weintraub D, Du Y, Buchsbaum R, Spencher HT, Hager M, Straka T, Bazil CW, Adams DJ, Resor SR Jr, Morrell MJ. Correlating lamotrigine serum concentrations with tolerability in patients with epilepsy. Neurology. 2004; 63:1022–1026.
Article157. Fröscher W, Keller F, Vogt H, Krämer G. Prospective study on concentration-efficacy and concentration-toxicity: correlations with lamotrigine serum levels. Epileptic Disord. 2002; 4:49–56.158. Franco V, Mazzucchelli I, Gatti G, Specchio LM, La Neve A, Papantonio A, Pzakaynakci AE, Perucca E. Changes in lamotrigine pharmacokinetics during pregnancy and the puerperium. Ther Drug Monit. 2008; 30:544–547.
Article159. Vidal E, Pascual C, Pou L. Determination of lamotrigine in human serum by liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999; 736:295–298.
Article160. Cheng CL, Chou CH, Hu OY. Determination of lamotrigine in small volumes of plasma by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 817:199–206.
Article161. Yamashita S, Furuno K, Kawasaki H, Gomita Y, Yoshinaga H, Yamatogi Y, Ohtahara S. Simple and rapid analysis of lamotrigine, a novel antiepileptic, in human serum by high-performance liquid chromatography using a solid-phase extraction technique. J Chromatogr B Biomed Appl. 1995; 670:354–357.
Article162. Biddlecombe RA, Dean KL, Smith CD, Jeal SC. Validation of a radioimmunoassay for the determination of human plasma concentrations of lamotrigine. J Pharm Biomed Anal. 1990; 8:691–694.
Article163. Sailstad JM, Findlay JW. Immunofluorometric assay for lamotrigine (Lamictal) in human plasma. Ther Drug Monit. 1991; 13:433–442.
Article164. Westley IS, Morris RG. Seradyn quantitative microsphere system lamotrigine immunoassay on a hitachi 911 analyzer compared with HPLC-UV. Ther Drug Monit. 2008; 30:634–637.
Article165. Shihabi ZK, Oles KS. Serum lamotrigine analysis by capillary electrophoresis. J Chromatogr B Biomed Appl. 1996; 683:119–123.
Article166. Theurillat R, Kuhn M, Thormann W. Therapeutic drug monitoring of lamotrigine using capillary electrophoresis. Evaluation of assay performance and quality assurance over a 4-year period in the routine arena. J Chromatogr A. 2002; 979:353–368.167. Watelle M, Demedts P, Franck F, De Deyn PP, Wauters A, Neels H. Analysis of the antiepileptic phenyltriazine compound lamotrigine using gas chromatography with nitrogen phosphorus detection. Ther Drug Monit. 1997; 19:460–464.
Article168. Dasgupta A, Hart AP. Lamotrigine analysis in plasma by gas chromatography-mass spectrometry after conversion to a tert-butyldimethylsilyl derivative. J Chromatogr B Biomed Sci Appl. 1997; 693:101–107.
Article169. Hallbach J, Vogel H, Guder WG. Determination of lamotrigine, carbamazepine and carbamazepine epoxide in human serum by gas chromatography mass spectrometry. Eur J Clin Chem Clin Biochem. 1997; 35:755–759.
Article170. Vermeij TA, Edelbroek PM. Robust isocratic high performance liquid chromatographic method for simultaneous determination of seven antiepileptic drugs including lamotrigine, oxcarbazepine and zonisamide in serum after solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 857:40–46.
Article171. Saracino MA, Koukopoulos A, Sani G, Amore M, Raggi MA. Simultaneous high-performance liquid chromatographic determination of olanzapine and lamotrigine in plasma of bipolar patients. Ther Drug Monit. 2007; 29:773–780.
Article172. Carrier DJ, Eckers C, Wolff JC. “In-source” fragmentation of an isobaric impurity of lamotrigine for its measurement by liquid chromatography tandem mass spectrometry after pre-concentration using solid phase extraction. J Pharm Biomed Anal. 2008; 47:731–737.
Article173. Lee W, Kim J, Kwon OH, Lee BI, Heo K. Determination of lamotrigine in human serum by high-performance liquid chromatography-tandem mass spectrometry. Neurol Sci. 2010; 31:717–720.
Article174. Shah HJ, Subbaiah G, Patel DM, Suhagia BN, Patel CN. Rapid quantification of lamotrigine in human plasma by two LC systems connected with tandem MS. J Chromatogr Sci. 2010; 48:375–381.
Article175. Nash EM, Sangha KS. Levetiracetam. Am J Health Syst Pharm. 2001; 58:1195–1199.
Article176. Ramael S, De Smedt F, Toublanc N, Otoul C, Boulanger P, Riethuisen JM, Stockis A. A Single-dose bioavailability of levetiracetam intravenous infusion relative to oral tablets and multiple-dose pharmacokinetics and tolerability of levetiracetam intravenous infusion compared with placebo in healthy subjects. Clin Ther. 2006; 28:734–744.177. Fay MA, Sheth RD, Gidal BE. Oral absorption kinetics of levetiracetam: the effect of mixing with food or enteral nutrition formulas. Clin Ther. 2005; 27:594–598.
Article178. Patsalos PN. Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet. 2004; 43:707–724.
Article179. Patsalos PN, Ghattaura S, Ratnaraj N, Sander JW. In situ metabolism of levetiracetam in blood of patients with epilepsy. Epilepsia. 2006; 47:1818–1821.
Article180. Grim SA, Ryan M, Miles MV, Tang PH, Strawsburg RH, deGrauw TJ, Fakhoury TA, Baumann RJ. Correlation of levetiracetam concentrations between serum and saliva. Ther Drug Monit. 2003; 25:61–66.
Article181. Mecarelli O, Li Voti P, Pro S, Romolo FS, Rotolo M, Rulitano P, Accornero N, Vanacore N. Saliva and serum levetiracetam concentrations in patients with epilepsy. Ther Drug Monit. 2007; 29:313–318.
Article182. Martens-Lobenhoffer J, Bode-Böger SM. Determination of levetiracetam in human plasma with minimal sample pretreatment. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 819:197–200.
Article183. Juenke J, Brown PI, Urry FM, McMillin GA. Drug monitoring and toxicology: a procedure for the monitoring of levetiracetam and zonisamide by HPLC-UV. J Anal Toxicol. 2006; 30:27–30.
Article184. Pucci V, Bugamelli F, Mandrioli R, Ferrranti A, Kenndler E, Raggi MA. High-performance liquid chromatographic determination of Levetiracetam in human plasma: comparison of different sample clean-up procedures. Biomed Chromatogr. 2004; 18:37–44.
Article185. Lancelin F, Franchon E, Kraoul L, Garciau I, Brovedani S, Tabaouti K, Landrė E, Chassoux F, Paubel P, Piketty ML. Therapeutic drug monitoring of levetiracetam by high-performance liquid chromatography with photodiode array ultraviolet detection: preliminary observations on correlation between plasma concentration and clinical response in patients with refractory epilepsy. Ther Drug Monit. 2007; 29:576–583.
Article186. Contin M, Mohamed S, Albani F, Riva R, Brauzzi A. Simple and validated HPLC-UV analysis of levetiracetam in deproteinized plasma of patients with epilepsy. J Chromatogr B Analyt Technol Biomed Life Sci. 2008; 18:129–132.
Article187. Vermeij TA, Edelbroek PM. High-performance liquid chromatographic and megabore gas-liquid chromatographic determination of levetiracetam (ucb L059) in human serum after solid-phase extraction. J Chromatogr B Biomed Appl. 1994; 662:134–139.
Article188. Isoherranen N, Roeder M, Soback S, Yagen B, Schurig V, Bialer M. Enantioselective analysis of levetiracetam and its enantiomer R-alpha-ethyl-2-oxo-pyrrolidine acetamide using gas chromatography and ion trap mass spectrometric detection. J Chromatogr B Biomed Sci Appl. 2000; 745:325–332.189. Jain DS, Subbaiah G, Sanyal M, Pal U, Shrivastav PS. Determination of levetiracetam in human plasma by liquid chromatography/electrospray tandem mass spectrometry and its application to bioequivalence studies. Rapid Commun Mass Spectrom. 2006; 20:2539–2547.
Article190. Guo T, Oswald LM, Mendu D, Soldin SJ. Determination of levetiracetam in human plasma/serum/saliva by liquid chromatography-electrospray tandem mass spectrometry. Clin Chim Acta. 2007; 375:115–118.
Article191. Matar KM. Quantification of levetiracetam in human plasma by liquid chromatography-tandem mass spectrometry: application to therapeutic drug monitoring. J Pharm Biomed Anal. 2008; 48:822–828.
Article192. Glauser TA. Oxcarbazepine in the treatment of epilepsy. Pharmacotherapy. 2001; 21:904–919.
Article193. Flesh G. Overview of the clinical pharmacokinetics of oxcarbazepine. Clin Drug Invest. 2004; 24:185–203.194. Keränen T, Jolkkonen J, Jensen PK, Menge GP, Andersson P. Absence of interaction between oxcarbazepine and erythromycin. Acta Neurol Scand. 1992; 86:120–123.
Article195. May TW, Korn-Merker E, Rambeck B. Clinical pharmacokinetics of oxcarbazepine. Clin Pharmacokinet. 2003; 42:1023–1042.
Article196. Benetello P, Furlanut M, Baraldo M, Tonon A, Furlanut M. Therapeutic drug monitoring of lamotrigine in patients suffering from resistant partial seizures. Eur Neurol. 2002; 48:200–203.
Article197. Bring P, Ensom MH. Does oxcarbazepine warrant therapeutic drug monitoring? A critical review. Clin Pharmacokinet. 2008; 47:767–778.198. Nirogi RV, Kandikere VN, Shukla M, Mudigonda K, Ajjala DR. Quantification of oxcarbazeipine and its active metabolite 10-hydroxycarbazepine in human plasma by high-performance liquid chromatography. Arzneimittelforschung. 2006; 56:517–523.199. Levert H, Odou P, Robert H. LC determination of oxcarbazepine and its active metabolite in human serum. J Pharm Biomed Anal. 2002; 28:517–525.
Article200. von Unruh GE, Paar WD. Gas chromatographic assay for oxcarbazepine and its main metabolites in plasma. J Chromatogr. 1985; 345:67–76.
Article201. Greiner-Sosanko E, Giannoutsos S, Lower DR, Virji MA, Krasowski MD. Drug monitoring: simultaneous analysis of lamotrigine, oxcarbazepine, 10-hydroxycarbazepine, and zonisamide by HPLC-UV and a rapid GC method using a nitrogen-phosphorus detector for levetiracetam. J Chromatogr Sci. 2007; 45:616–622.
Article202. Flesch G, Francotte E, Hell F, Degen PH. Determination of the R-(–) and S-(+) enantiomers of the monohydroxylated metabolite of oxcarbazepine in human plasma by enantio-selective high-performance liquid chromatography. J Chromatogr. 1992; 581:147–151.
Article203. Mazzucchelli I, Franco V, Fattore C, Marchiselli R, Perucca E, Gatti G. A novel enantioselective microassay for the high-performance liquid chromatography determination of oxcarbazepine and its active metabolite monohydroxycarbazepine in human plasma. Ther Drug Monit. 2007; 29:319–324.
Article204. Schachter SC. Tiagabine. Epilepsia. 1999; 40:S17–S22.
Article205. Gustavson LE, Mengel HB. Pharmacokinetics of tiagabine, a gamma-aminobutyric acid-uptake inhibitor, in healthy subjects after single and multiple doses. Epilepsia. 1995; 36:605–611.
Article206. Wilson JF, Tsanaclis LM, Williams J, Tedstone JE, Richens A. Evaluation of assay techniques for the measurement of antiepileptic drugs in serum: a study based on external quality assurance measurements. Ther Drug Monit. 1989; 11:185–195.207. Williams J, Bialer M, Johannessen SI, Kramer G, Levy R, Mattson RH, Perucca E, Patsalos PN, Wilson JF. Interlaboratory variability in the quantification of new generation antiepileptic drugs based on external quality assessment data. Epilepsia. 2003; 44:40–45.208. Chollet DF, Castella E, Goumaz L, Anderegg G. Gas chromatography-mass spectrometry assay method for the therapeutic drug monitoring of the antiepileptic drug tiagabine. J Pharm Biomed Anal. 1999; 21:641–646.
Article209. Gustavson LE, Chu SY. High-performance liquid chromatographic procedure for the determination of tiagabine concentrations in human plasma using electrochemical detection. J Chromatogr. 1992; 574:313–318.
Article210. Sachdeo RC. Topiramate: Clinical profile in epilepsy. Clin Pharmacokinet. 1998; 34:335–346.211. Bialer M, Doose DR, Murthy B, Curtin C, Wang SS, Twyman RE, Schwabe S. Pharmacokinetic interactions of topiramate. Clin Pharmacokinet. 2004; 43:763–800.
Article212. Sachdeo RC, Sachdeo SK, Walker SA, Kramer LD, Nayak RK, Doose DR. Steady-state pharmacokinetics of topiramate and carbamazepine in patients with epilepsy during monotherapy and concomitant therapy. Epilepsia. 1996; 37:774–780.
Article213. Rosenfeld WE, Liao S, Kramer LD, Anderson G, Palmer M, Levy RH, Nayak RK. Comparison of the steady-state pharmacokinetics of topiramate and valproate in patients with epilepsy during monotherapy and concomitant therapy. Epilepsia. 1997; 38:324–333.
Article214. Reife RA, Pledger G, Doose DR. Relationship of steady-state plasma topiramate (Topamax) concentration to clinical efficacy and tolerability. Epilepsia. 1995; 36(Suppl 3):152.215. Contin M, Riva R, Albani F, Avoni P, Baruzzi A. Topiramate therapeutic monitoring in patients with epilepsy: effect of concomitant antiepileptic drugs. Ther Drug Monit. 2002; 24:332–337.
Article216. Lhatoo SD, Wong IC, Sander JW. Prognostic factors affecting long-term retention of topiramate in patients with chronic epilepsy. Epilepsia. 2000; 41:338–341.
Article217. Holland ML, Uetz JA, Ng KT. Automated capillary gas chromatographic assay using flame ionization detection for the determination of topiramate in plasma. J Chromatogr. 1988; 433:276–281.
Article218. Riffitts JM, Gisclon LG, Stubbs RJ, Palmer ME. A capillary gas chromatographic assay with nitrogen phosphorus detection for the quantification of topiramate in human plasma, urine and whole blood. J Pharm Biomed Anal. 1999; 19:363–371.
Article219. Berry DJ, Patsalos PN. Comparison of topiramate concentrations in plasma and serum by fluorescence polarization immunoassay. Ther Drug Monit. 2000; 22:460–464.
Article220. la Marca G, Malvagia S, Filippi L, Fiorini P, Innocenti M, Luceri F, Pieraccini G, Moneti G, Francese S, Dani FR, Guerrini R. Rapid assay of topiramate in dried blood spots by a new liquid chromatography-tandem mass spectrometric method. J Pharm Biomed Anal. 2008; 48:1392–1396.
Article221. Tang PH, Miles MV, Glauser TA, Coletta L, Doughman N, Doose D, Frey M, DeGrauw A. An improved gas chromatography assay for topiramate monitoring in pediatric patients. Ther Drug Monit. 2000; 22:195–201.
Article222. Chen S, Carvey P. Validation of liquid-liquid extraction followed by flow-injection negative ion electrospray mass spectrometry assay to topiramate in human plasma. Rapid Commun Mass Spectrom. 2001; 15:159–163.
Article223. Contin M, Riva R, Albani F, Baruzzi A. Simple and rapid liquid chromatographic-turbo ion spray mass spectrophotometric determination of topiramate in human plasma. J Chromatogr B Biomed Sci Appl. 2001; 761:133–137.224. Christensen J, H⊘jskov CS, Poulsen JH. Liquid chromatography tandem mass spectrometry assay for topiramate in plasma and cerbrospinal fluid: validation and comparison with fluorescence-polarization immunoassay. Ther Drug Monit. 2002; 24:658–664.225. Park JH, Park YS, Lee MH, Rhim SY, Song JC, Lee SJ, Kim JM, Shaw LM, Kang JS. Determination of plasma topiramate concentration using LC-MS/MS for pharmacokinetic and bioequivalence studies in healthy Korean volunteers. Biomed Chromatogr. 2008; 22:822–829.
Article226. Grant SM, Hell RC. Vigabatrin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in epilepsy and disorders of motor control. Drugs. 1991; 41:889–926.227. Kälviäinen R, Nousianen I. Visual field defects with vigabatrin: epidemiology and therapeutic implications. CNS Drugs. 2001; 15:217–230.228. Chiron C, Dulac O. Drug therapy for West's syndrome. Adv Exp Med Biol. 2002; 497:51–56.
Article229. Riikonen R. The latest on infantile spasms. Curr Opin Neurol. 2005; 18:91–95.
Article230. Curatolo P, Bombardieri R, Cerminara C. Current management for epilepsy in tuberous sclerosis complex. Curr Opin Neurol. 2006; 19:119–123.
Article231. Rey E, Pons G, Olive G. Vigabatrin. Clinical pharmacokinetics. Clin Pharmacokinet. 1992; 23:267–278.232. Franco V, Mazzucchelli I, Fattore C, Marchiselli R, Gatti G, Perucca E. Stereoselective determination of vigabatrin enantiomers in human plasma by high performance liquid chromatography using UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 854:63–37.
Article233. Hsieh CY, Wang SY, Kwan AL, Wu HL. Fluorescent high-performance liquid chromatographic analysis of vigabatrin enantiomers after derivatizing with naproxen acyl chloride. J Chromatogr A. 2008; 1178:166–170.
Article234. Rey E, Pons G, Richard MO, Vauzelle F, D'Athis P, Chiron C, Dulac O, Beaumont D, Olive G. Pharmacokinetics of the individual enantiomers of vigabatrin (gamma-vinyl GABA) in epileptic children. Br J Clin Pharmacol. 1990; 30:253–257.235. Haegele KD, Schechter PJ. Kinetics of the enantiomers of vigabatrin after an oral dose of the racemate or the active S-enantiomer. Clin Pharmacol Ther. 1986; 40:581–586.
Article236. Grove J, Alken RG, Schechter PJ. Assay of gamma-vinylgamma-aminobutyric acid (4-amino-hex-5-enoic acid) in plasma and urine by automatic amino acid analysis. Application to human pharmacokinetics. J Chromatogr. 1984; 306:383–387.237. Smithers JA, Lang JF, Okerholm RA. Quantitative analysis of vigabatrin in plasma and urine by reversed-phase high-performance liquid chromatography. J Chromatogr. 1985; 341:232–238.
Article238. Haegele KD, Schoun J, Alken RG, Huebert ND. Determination of the R(–)- and S(+)-enantiomers of gamma-vinylgamma-aminobutyric acid in human body fluids by gas chromatography-mass spectrometry. J Chromatogr. 1983; 274:103–110.239. Chen TM, Contario JJ. High-peformance liquid chromatographic resolution of enantiomers of gamma-vinyl-gamma-aminobutyric acid. J Chromatogr. 1984; 314:495–498.240. Brückner H, Wittner R, Godel H. Automated enantioseparation of amino acids by derivatization with o-phthaldialdehyde and N-acylated cysteines. J Chromatogr. 1989; 476:73–82.
Article241. Vermeij TA, Edelbroek PM. High-performance liquid chromatographic analysis of vigabatrin enantiomers in human serum by precolumn derivatization with o-phthaldialdehyde-N-acetyl-L-cysteine and fluorescence detection. J Chromatogr B Biomed Sci Appl. 1998; 716:233–238.
Article242. Chollet DF, Goumaz L, Juliano C, Anderegg G. Fast isocratic high-performance liquid chromatographic assay method for the simultaneous determination of gabapentin and vigabatrin in human serum. J Chromatogr B Biomed Sci Appl. 2000; 746:311–314.
Article243. Ratnaraj N, Patsalos PN. A high-performance liquid chromatography micromethod for the simultaneous determination of vigabatrin and gabapentin in serum. Ther Drug Monit. 1998; 20:430–434.
Article244. Porter RJ. Mechanisms of action of new antiepileptic drugs. Epilepsia. 1989; 30 Suppl. 1:S29–34.
Article245. Sackellares JC, Donofrio PD, Wagner JG, Abou-Khalil B, Berent S, Aasved-Hoyt K. Pilot study of zonisamide (1,2-benzisoxazole-3-methanesulfonamide) in patients with refractory partial seizures. Epilepsia. 1985; 26:206–211.
Article246. Wirth HJ, Unger KK, Hearn MT. High-performance liquid chromatography of amino acids, peptides and proteins. CIX. Investigations on the relation between the ligand density of cibacron blue immobilized porous and non-porous sorbents and protein-binding capacities and association constants. J Chromatogr. 1991; 550:383–395.247. de Collongue-Poyet B, Vidal-Madjar C, Sebille B, Unger KK. Study of conformational effects of recombinant interferon-gamma adsorbed on a non-porous reversed-phase silica support. J Chromatogr B Biomed Appl. 1995; 664:155–161.248. Paasch BD, Lin YS, Porter S, Mondi NB, Barder TJ. Determination of Ro 48–3656 in rat plasma by reversed-phase high-performance liquid chromatography: Comparison of 1.5-microm nonporous silica to 3.5-microm porous silica analytical columns. J Chromatogr B Biomed Sci Appl. 1997; 704:231–242.249. Furuno K, Oishi R, Gomita Y, Eto K. Simple and sensitive assay of zonisamide in human serum by high-performance liquid chromatography using a solid-phase extraction technique. J Chromatogr B Biomed Appl. 1994; 656:456–459.
Article250. Juergens U. Simultaneous determiantion of zonisamide and nine other anti-epileptic drugs and metabolites in serum. A comparison of microbore and conventional high-performance liquid chromatography. J Chromatogr. 1987; 385:233–240.251. Shimoyama R, Ohkubo T, Sugawara K. Monitoring of zonisamide in human breast milk and maternal plasma by solid-phase extraction HPLC method. Biomed Chromatogr. 1999; 13:370–372.
Article252. Makino K, Goto Y, Sueyasu M, Futagami K, Kataoka Y, Oishi R. Micellar electrokinetic capillary chromatography for therapeutic drug monitoring of zonisamide. J Chromatogr B Biomed Sci Appl. 1997; 695:417–425.
Article253. Kalbe K, Nishimura S, Ishii H, Sunahara N, Naruto S, Kurooka S. Competitive binding enzyme immunoassay for zonisamide, a new antiepileptic drug, with selected pairedenzyme labeled antigen and antibody. Clin Chem. 1990; 36:24–27.
Article