Korean J Physiol Pharmacol.
2010 Jun;14(3):145-150. 10.4196/kjpp.2010.14.3.145.
Regulation of Adenosine-activated GIRK Channels by Gq-coupled Receptors in Mouse Atrial Myocytes
- Affiliations
-
- 1Department of Physiology, Sungkyunkwan University School of Medicine, Suwon 440-746, Korea. hanacho@med.skku.ac.kr
- KMID: 2285395
- DOI: http://doi.org/10.4196/kjpp.2010.14.3.145
Abstract
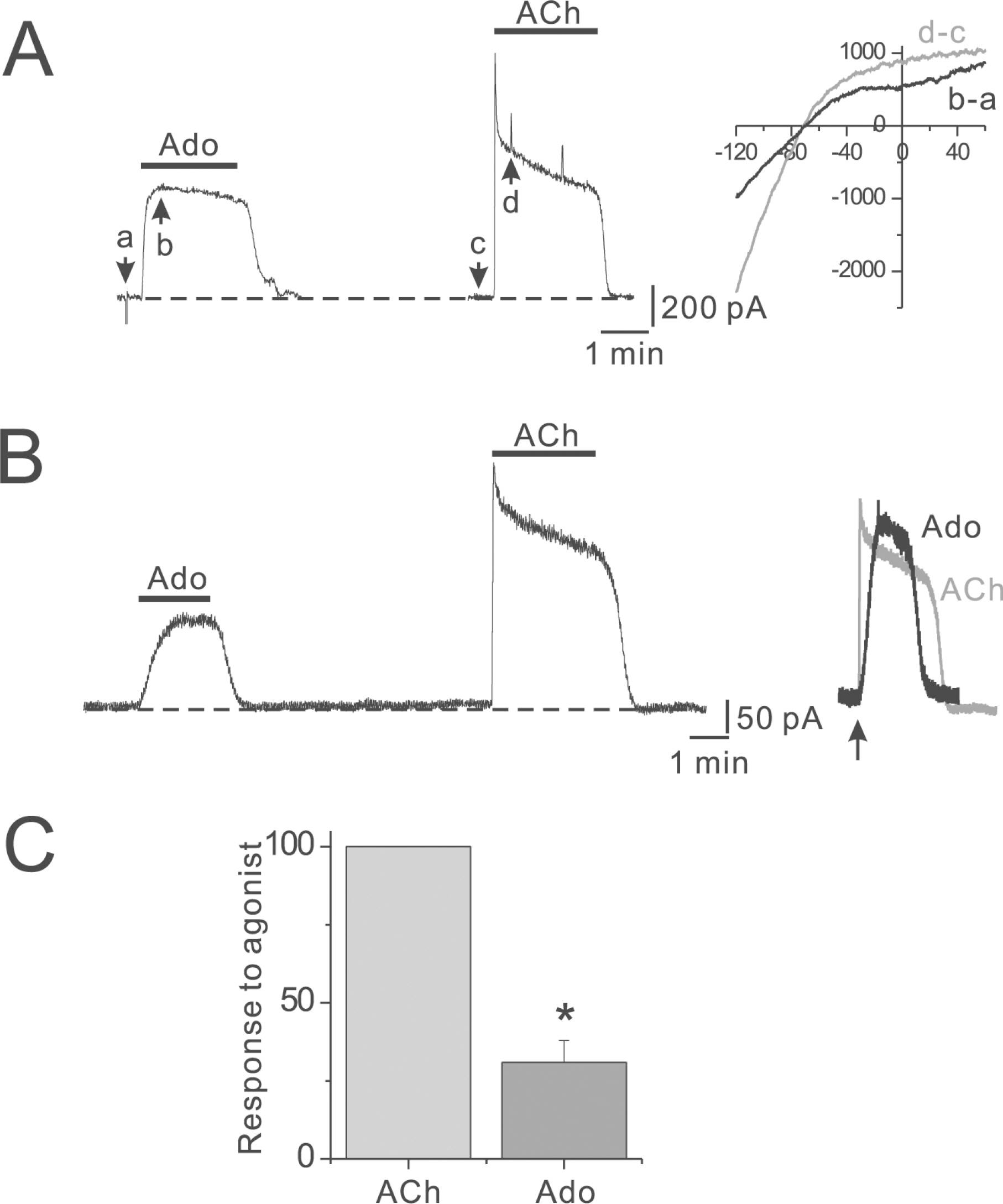
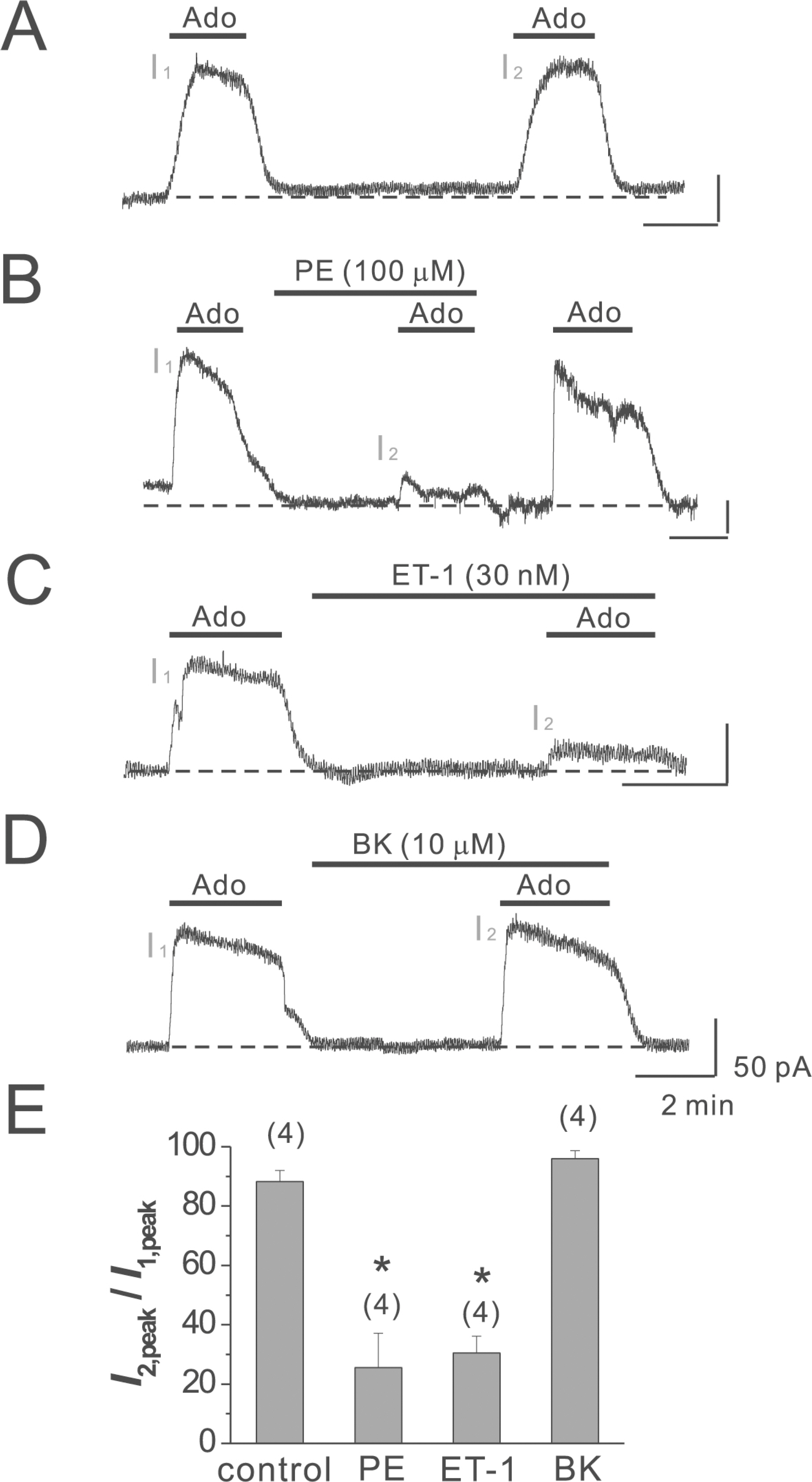
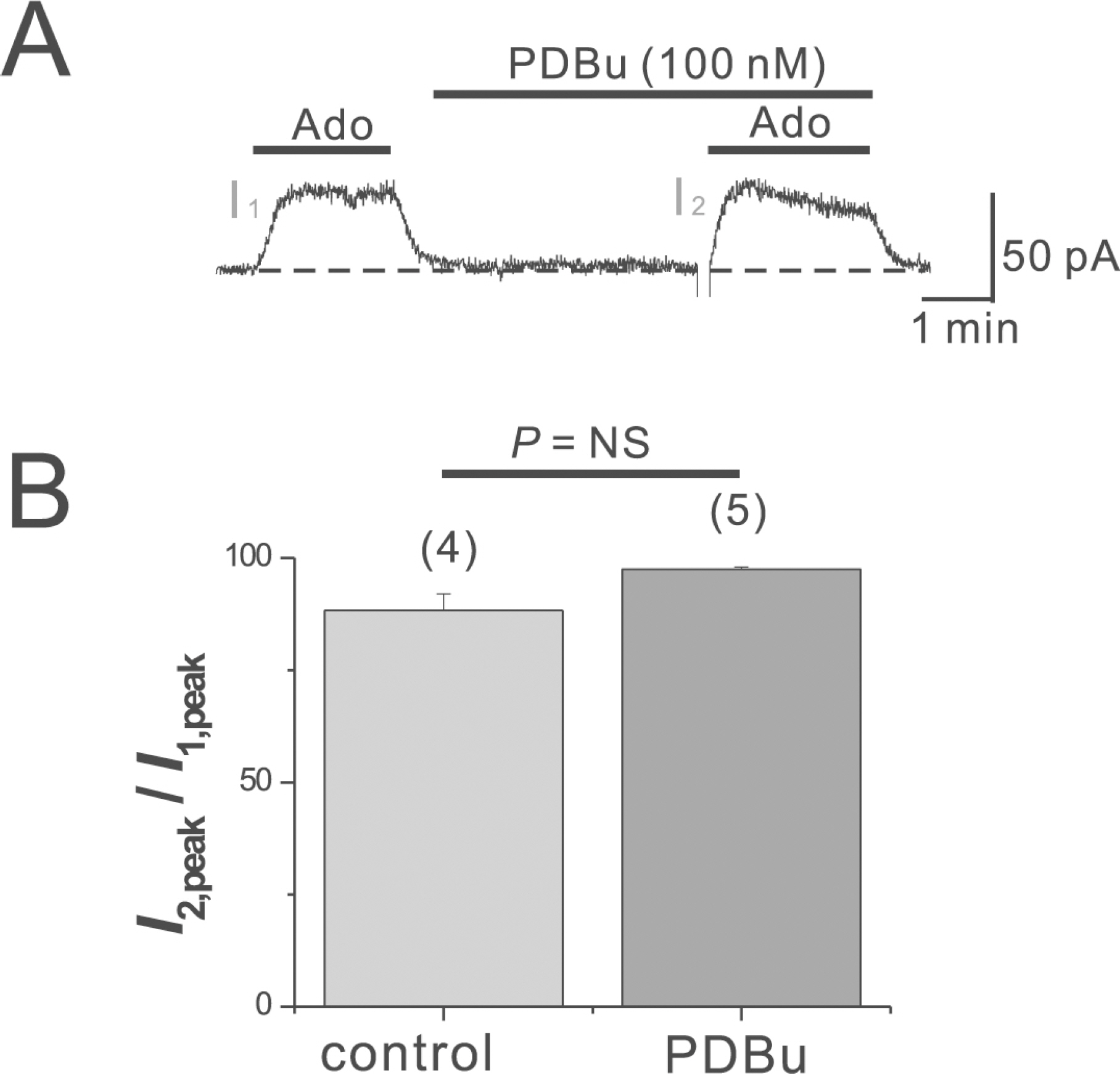
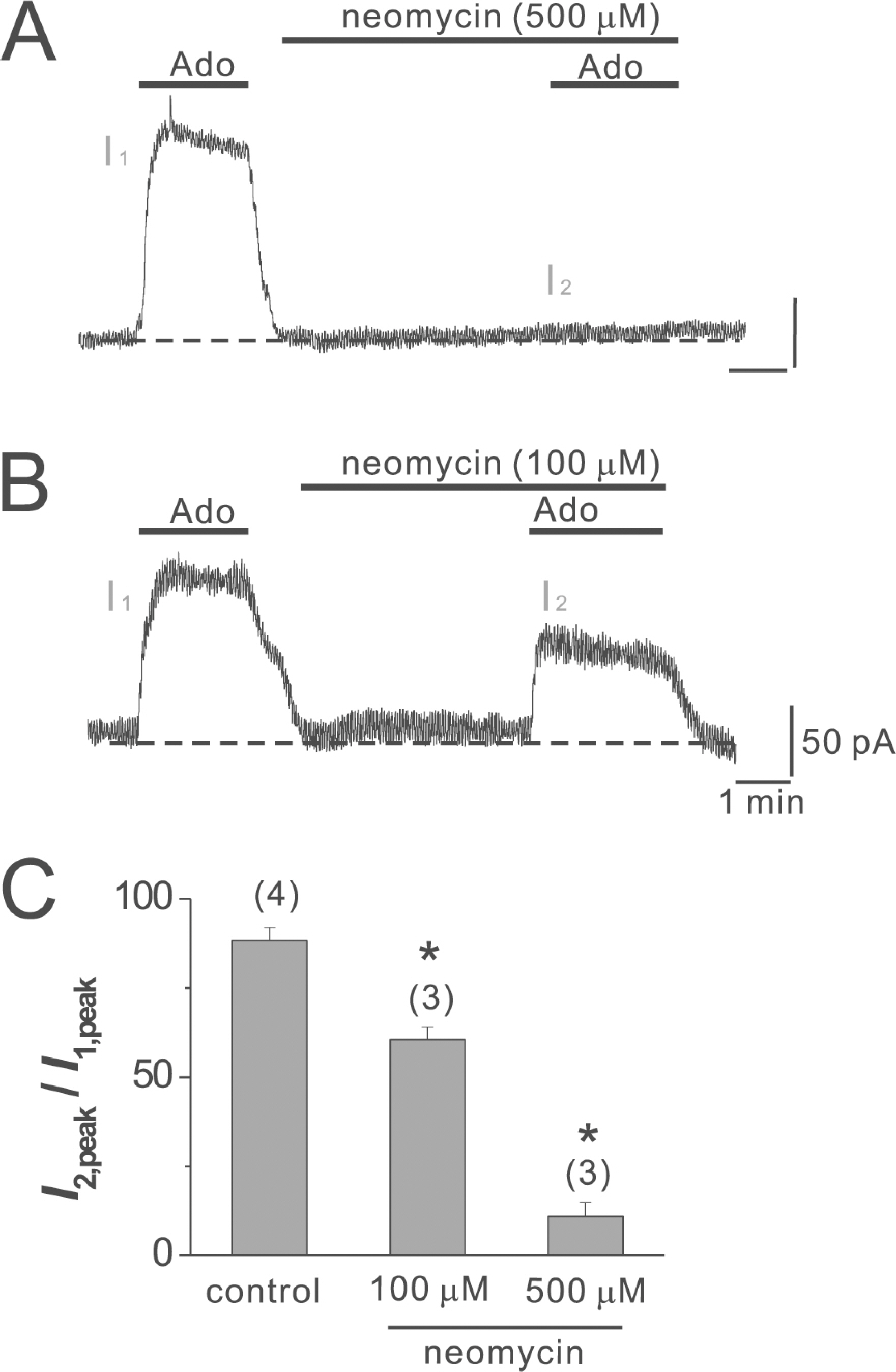
- Adenosine (Ado) is an important mediator of the endogenous defense against ischemia-induced injury in the heart. The action of Ado is mediated by activation of G protein-gated inwardly rectifying K+ (GIRK) channels. In turn, GIRK channels are inhibited by reducing phosphatidylinositol 4,5-bisphosphate (PIP2) through Gq protein-coupled receptors (GqPCRs). We previously found that GIRK channels activated by acetylcholine, a muscarinic M2 acetylcholine receptor agonist, are inhibited by GqPCRs in a receptor-specific manner. However, it is not known whether GIRK channels activated by Ado signaling are also regulated by GqPCRs. Presently, this was investigated in mouse atrial myocytes using the patch clamp technique. GIRK channels were activated by 100 micrometer Ado. When Ado was repetitively applied at intervals of 5~6 min, the amplitude of second Ado-activated GIRK currents (I(K(Ado))) was 88.3+/-3.7% of the first I(K(Ado)) in the control. Pretreatment of atrial myocytes with phenylephrine, endothelin-1, or bradykinin prior to a second application of Ado reduced the amplitude of the second I(K(Ado)) to 25.5+/-11.6%, 30.5+/-5.6%, and 96.0+/-2.7%, respectively. The potency of I(K(Ado)) inhibition by GqPCRs was different with that observed in acetylcholine-activated GIRK currents (I(K(ACh))) (endothelin-1>phenylephrine>bradykinin). I(K(Ado)) was almost completely inhibited by 500 micrometer of the PIP2 scavenger neomycin, suggesting low PIP2 affinity of I(K(Ado)). Taken together, these results suggest that the crosstalk between GqPCRs and the Ado-induced signaling pathway is receptor-specific. The differential change in PIP2 affinity of GIRK channels activated by Ado and ACh may underlie, at least in part, their differential responses to GqPCR agonists.
MeSH Terms
Figure
Reference
-
References
1. Babbitt DG, Virmani R, Forman MB. Intracoronary adenosine administered after reperfusion limits vascular injury after prolonged ischemia in the canine model. Circulation. 1989; 80:1388–1399.
Article2. Ely SW, Berne RM. Protective effects of adenosine in myocardial ischemia. Circulation. 1992; 85:893–904.
Article3. Gross GJ. ATP-sensitive potassium channels and myocardial preconditioning. Basic Res Cardiol. 1995; 90:85–88.
Article4. Li GC, Vasquez JA, Gallagher KP, Lucchesi BR. Myocardial protection with preconditioning. Circulation. 1990; 82:609–619.
Article5. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986; 74:1124–1136.
Article6. Olafsson B, Forman MB, Puett DW, Pou A, Cates CU, Friesinger GC, Virmani R. Reduction of reperfusion injury in the canine preparation by intracoronary adenosine: importance of the endothelium and the no-reflow phenomenon. Circulation. 1987; 76:1135–1145.
Article7. Ely SW, Mentzer RM Jr, Lasley RD, Lee BK, Berne RM. Functional and metabolic evidence of enhanced myocardial tolerance to ischemia and reperfusion with adenosine. J Thorac Cardiovasc Surg. 1985; 90:549–556.
Article8. Reibel DK, Rovetto MJ. Myocardial ATP synthesis and mechanical function following oxygen deficiency. Am J Physiol. 1978; 234:H620–624.
Article9. Matherne GP, Linden J, Byford AM, Gauthier NS, Headrick JP. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc Natl Acad Sci USA. 1997; 94:6541–6546.
Article10. Belardinelli L, Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983; 244:H734–737.
Article11. Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995; 9:359–365.
Article12. Bunemann M, Pott L. Down-regulation of A1 adenosine receptors coupled to muscarinic K+ current in cultured guineapig atrial myocytes. J Physiol. 1995; 482:81–92.13. Kurachi Y, Nakajima T, Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986; 407:264–274.14. Wellner-Kienitz MC, Bender K, Meyer T, Bunemann M, Pott L. Overexpressed A(1) adenosine receptors reduce activation of acetylcholine-sensitive K+ current by native muscarinic M(2) receptors in rat atrial myocytes. Circ Res. 2000; 86:643–648.15. Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995; 374:135–141.16. Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993; 364:802–806.
Article17. Sakmann B, Noma A, Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983; 303:250–253.18. Kobayashi T, Ikeda K. G protein-activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des. 2006; 12:4513–4523.
Article19. Yamada M. The role of muscarinic K+ channels in the negative chronotropic effect of a muscarinic agonist. J Pharmacol Exp Ther. 2002; 300:681–687.20. Ho IH, Murrell-Lagnado RD. Molecular mechanism for sodium-dependent activation of G protein-gated K+ channels. J Physiol. 1999; 520 Pt. 3:645–651.21. Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998; 391:803–806.22. Sui JL, Petit-Jacques J, Logothetis DE. Activation of the atrial KACh channel by the betagamma subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci USA. 1998; 95:1307–1312.23. Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol. 1999; 1:183–188.24. Jenkinson S, Nahorski SR, Challiss RA. Disruption by lithium of phosphatidylinositol-4,5-bisphosphate supply and inositol-1,4,5-trisphosphate generation in Chinese hamster ovary cells expressing human recombinant m1 muscarinic receptors. Mol Pharmacol. 1994; 46:1138–1148.25. Willars GB, Nahorski SR, Challiss RA. Differential regulation of muscarinic acetylcholine receptor-sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. J Biol Chem. 1998; 273:5037–5046.
Article26. Cho H, Nam GB, Lee SH, Earm YE, Ho WK. Phosphatidylinositol 4,5-bisphosphate is acting as a signal molecule in alpha(1)-adrenergic pathway via the modulation of acetylcholine-activated K+ channels in mouse atrial myocytes. J Biol Chem. 2001; 276:159–164.27. Cho H, Lee D, Lee SH, Ho WK. Receptor-induced depletion of phosphatidylinositol 4,5-bisphosphate inhibits inwardly rectifying K+ channels in a receptor-specific manner. Proc Natl Acad Sci USA. 2005; 102:4643–4648.28. Clerk A, Sugden PH. Regulation of phospholipases C and D in rat ventricular myocytes: stimulation by endothelin-1, bradykinin and phenylephrine. J Mol Cell Cardiol. 1997; 29:1593–1604.
Article29. Cho H, Hwang JY, Kim D, Shin HS, Kim Y, Earm YE, Ho WK. Acetylcholine-induced phosphatidylinositol 4,5-bisphosphate depletion does not cause short-term desensitization of G protein-gated inwardly rectifying K+ current in mouse atrial myocytes. J Biol Chem. 2002; 277:27742–27747.30. Sohn JW, Lee D, Cho H, Lim W, Shin HS, Lee SH, Ho WK. Receptor-specific inhibition of GABAB-activated K+ currents by muscarinic and metabotropic glutamate receptors in immature rat hippocampus. J Physiol. 2007; 580:411–422.31. Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J Biol Chem. 2004; 279:37271–37281.
Article32. Arbuzova A, Martushova K, Hangyas-Mihalyne G, Morris AJ, Ozaki S, Prestwich GD, McLaughlin S. Fluorescently labeled neomycin as a probe of phosphatidylinositol-4,5-bisphosphate in membranes. Biochim Biophys Acta. 2000; 1464:35–48.
Article33. Schulze D, Krauter T, Fritzenschaft H, Soom M, Baukrowitz T. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulation of ATP and pH sensitivity in Kir channels. A tale of an active and a silent PIP2 site in the N terminus. J Biol Chem. 2003; 278:10500–10505.34. Cho H, Youm JB, Earm YE, Ho WK. Inhibition of acetylcholine-activated K+ current by chelerythrine and bisindolylmaleimide I in atrial myocytes from mice. Eur J Pharmacol. 2001; 424:173–178.35. Sohn JW, Lim A, Lee SH, Ho WK. Decrease in PIP(2) channel interactions is the final common mechanism involved in PKC-and arachidonic acid-mediated inhibitions of GABA(B)-activated K+ current. J Physiol. 2007; 582:1037–1046.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Modulation of Muscarinic K+ Channel by Protein Kinase C in Ischemic Rat Atrial Myocytes
- Stretch-activated K+ Channels in Rat Atrial Myocytes
- Presynaptic Mechanism Underlying Regulation of Transmitter Release by G Protein Coupled Receptors
- Mechanisms involved in adenosine pharmacological preconditioning-induced cardioprotection
- Molecular Vibration-Activity Relationship in the Agonism of Adenosine Receptors