Korean J Physiol Pharmacol.
2009 Feb;13(1):23-26. 10.4196/kjpp.2009.13.1.23.
The Effect of Extracellular Glutamate Release on Repetitive Transient Ischemic Injury in Global Ischemia Model
- Affiliations
-
- 1Department of Biomedical Engineering, School of Medicine, Kyunghee University, Seoul 130-702, Korea. sigmoidus@khu.ac.kr
- 2Healthcare Industry Research Institute, Kyunghee University, Seoul 130-702, Korea.
- 3Department of Neurosurgery, Kyunghee University Medical Center, Seoul 130-702, Korea.
- 4Program of Medical Engineering, Kyunghee University, Seoul 130-702, Korea.
- KMID: 2285367
- DOI: http://doi.org/10.4196/kjpp.2009.13.1.23
Abstract
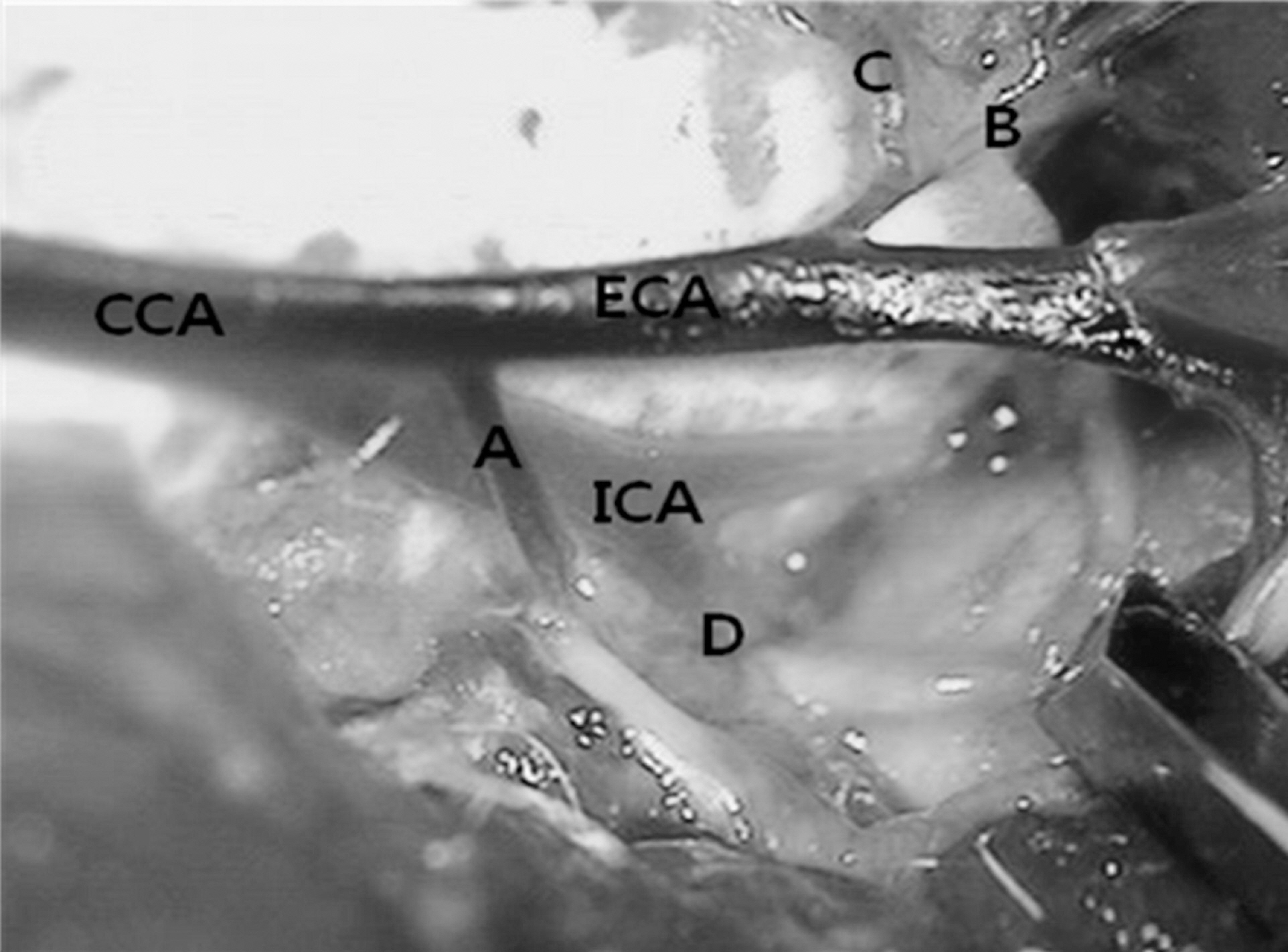
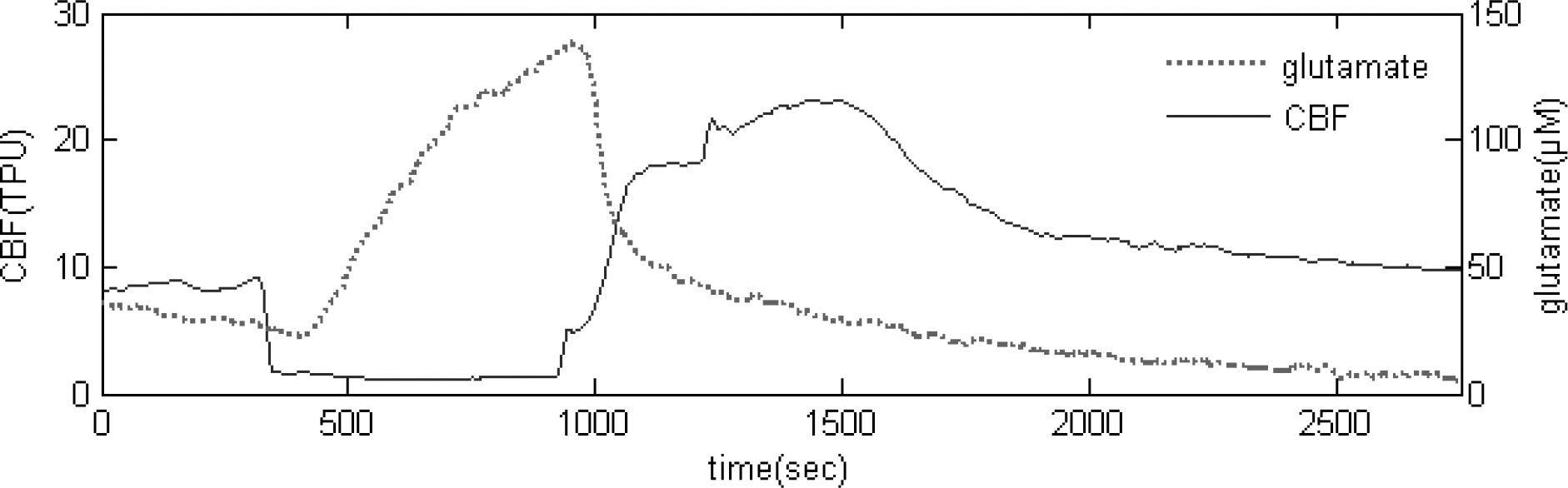
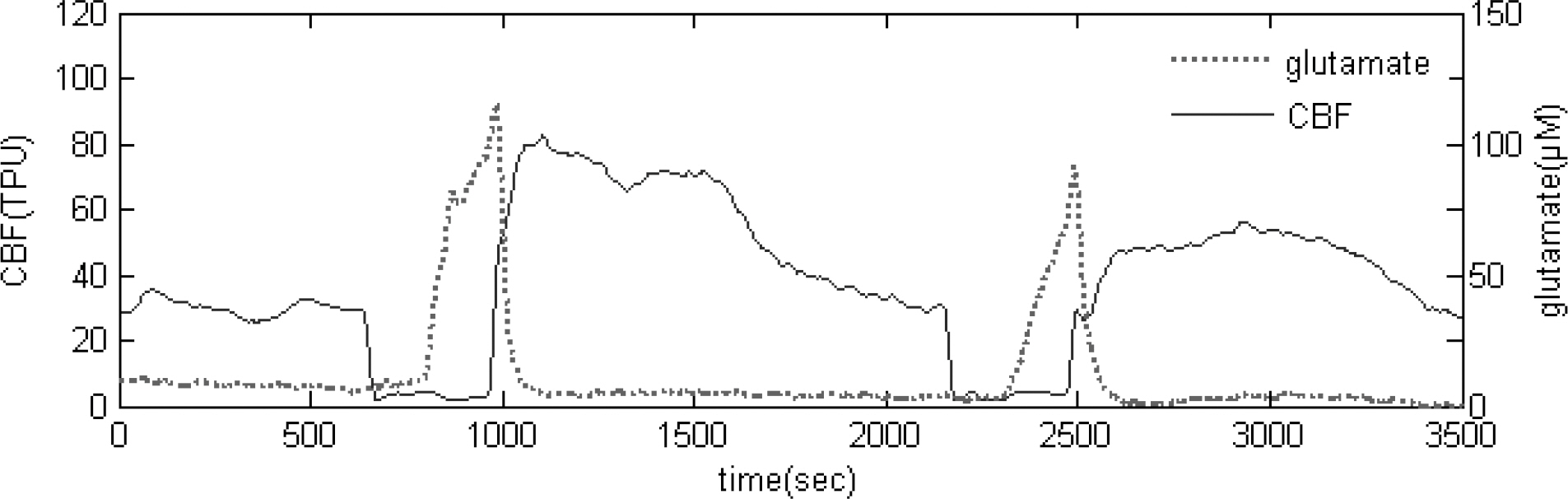
- During operations, neurosurgeons usually perform multiple temporary occlusions of parental artery, possibly resulting in the neuronal damage. It is generally thought that neuronal damage by cerebral ischemia is associated with extracellular concentrations of the excitatory amino acids. In this study, we measured the dynamics of extracellular glutamate release in 11 vessel occlusion (VO) model to compare between single occlusion and repeated transient occlusions within short interval. Changes in cerebral blood flow were monitored by laser-Doppler flowmetry simultaneously with cortical glutamate level measured by amperometric biosensor. From real time monitoring of glutamate release in 11 VO model, the change of extracellular glutamate level in repeated transient occlusion group was smaller than that of single occlusion group, and the onset time of glutamate release in the second ischemic episode of repeated occlusion group was delayed compared to the first ischemic episode which was similar to that of single 10 min ischemic episode. These results suggested that repeated transient occlusion induces less glutamate release from neuronal cell than single occlusion, and the delayed onset time of glutamate release is attributed to endogeneous protective mechanism of ischemic tolerance.
MeSH Terms
Figure
Reference
-
Blanco M., Lizasoain I., Sobrino T., Vivancos J., Castillo J. Ischemic preconditioning: a novel target for neuroprotective therapy. Cerebrovasc Dis. 21(Suppl 2):38–47. 2006.
ArticleDowden J., Corbett D. Ischemic preconditioning in 18 to 20 month-old gerbils long-term survival with functional outcome measures. Stroke. 30:1240–1246. 1999.Katayama Y., Kawamata T., Tamura T., Hovda DA., Becker DP., Tsubokawa T. Calcium-dependent glutamate release concomitant with massive potassium flux during cerebral ischemia in vivo. Brain Res. 558:136–140. 1991.
ArticleKirino T., Tsijita Y., Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab. 11:299–307. 1991.
ArticleKitagawa K., Matsumoto M., Kuwabara K., Tagaya M., Ohtsuki T., Hata R., Ueda H., Handa N., Kimura K., Kamada T. Ischemic tolerance phenomenon detected in various brain regions. Brain Res. 561:203–211. 1991.
ArticleKristial T., Siesjö BK. Calcium in ischemic cell death. Stroke. 29:705–718. 1998.
ArticleLee JM., Grabb MC., Zipfel GJ., Choi DW. Brain tissue responses to ischemia. J Clin Investigat. 106:723–773. 2000.
ArticleLimbrick DD Jr. Sombati S, DeLorenzo RJ. Calcium influx constitutes the ionic basis for the maintenance of glutamate-induced extended neuronal depolarization associated with hippocampal neuronal death. Cell Calcium. 33:69–81. 2003.Lin B., Globus MY., Dietrich WD., Busto R., Martinez E., Ginsberg MD. Differing neurochemical and morphological sequelae of global ischemia: comparison of single- and multiple- insult paradigms. J Neurochem. 59:2213–2223. 1992.Lin CH., Chen PS., Gean PW. Glutamate preconditioning prevents neuronal death induced by combined oxygen-glucose deprivation in cultured cortical neurons. Eur J Phamacol. 589:85–93. 2008.
ArticleNakata N., Kato H., Kogure K. Effects of repeated cerebral ischemia on extracellular amino acid concentrations measured with intracerebral microdialysis in the gerbil hippocampus. Stroke. 24:458–463. 1993.
ArticleSchaller B., Graf R. Cerebral ischemic preconditioning: an experimental phenomenon or a clinical important entity of stroke prevention? J Neurol. 249:1503–1511. 2002.Shimazaki K., Nakamura K., Nakamura K., Oquro K., Masuzawa T., Kudo Y., Kawai N. Reduced calcium elevation in hippocampal CA1 neurons of ischemia tolerant gerbils. Neuroreport. 9:1875–1878. 1998.Tomida S., Nowak TS Jr., Vass K., Lohr JM., Klatzo I. Experimental model for repetitive ischemic attacks in the gerbil: The cumulative effect of repeated ischemic insults. J Cereb Blood Flow Metab. 7:773–782. 1987.
ArticleUeda Y., Obrenovitch TP., Lok SY., Sarna GS., Symon L. Changes in extracellular glutamate concentration produced in the rat striatum by repeated ischemia. Stroke. 23:1125–1131. 1992.
ArticleZhao H., Asai S., Kanematsu K., Kunimatsu T., Khno T., Ishikawa K. Real-time monitoring of the effects of normothermia on extracellular glutamate re-uptake in the rat following global brain ischemia. Neuroreport. 8:2389–2393. 1997.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective Effects by Nimodipine Treatment in the Experimental Global Ischemic Rat Model : Real Time Estimation of Glutamate
- Effect of Hyperglycemia on Glutamate , Glycine and Aspartate Concentrations during Peri-ischemic Periods in the Rabbit Hippocampus
- The Change of Taurine in The Transient Global Ischemic Rabbit
- Effect of hypocapnia on extracellular glutamate and glycine concentrations during peri-ischemic period in the rabbit hippocampus
- Animal Model of Cerebral Ischemia