Healthc Inform Res.
2014 Apr;20(2):109-116. 10.4258/hir.2014.20.2.109.
Characteristics Desired in Clinical Data Warehouse for Biomedical Research
- Affiliations
-
- 1Department of Biomedical Informatics, Asan Medical Center, Seoul, Korea. rufiji@gmail.com
- 2Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Emergency Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Division of General Internal Medicine, Brigham and Women's Hospital, Boston, MA, USA.
- KMID: 2284570
- DOI: http://doi.org/10.4258/hir.2014.20.2.109
Abstract
OBJECTIVES
Due to the unique characteristics of clinical data, clinical data warehouses (CDWs) have not been successful so far. Specifically, the use of CDWs for biomedical research has been relatively unsuccessful thus far. The characteristics necessary for the successful implementation and operation of a CDW for biomedical research have not clearly defined yet.
METHODS
Three examples of CDWs were reviewed: a multipurpose CDW in a hospital, a CDW for independent multi-institutional research, and a CDW for research use in an institution. After reviewing the three CDW examples, we propose some key characteristics needed in a CDW for biomedical research.
RESULTS
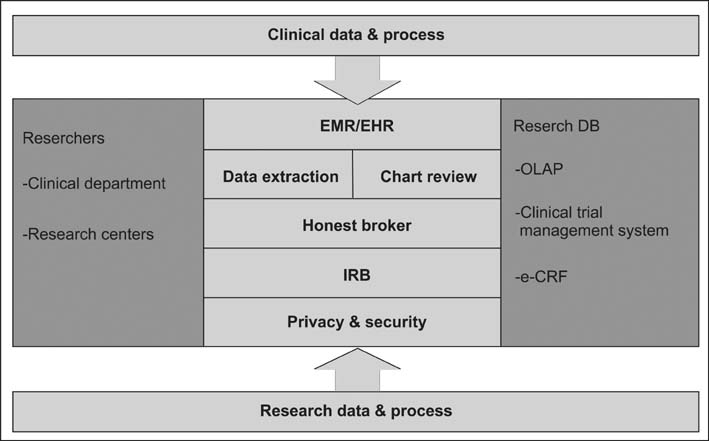
A CDW for research should include an honest broker system and an Institutional Review Board approval interface to comply with governmental regulations. It should also include a simple query interface, an anonymized data review tool, and a data extraction tool. Also, it should be a biomedical research platform for data repository use as well as data analysis.
CONCLUSIONS
The proposed characteristics desired in a CDW may have limited transfer value to organizations in other countries. However, these analysis results are still valid in Korea, and we have developed clinical research data warehouse based on these desiderata.
Keyword
MeSH Terms
Figure
Reference
-
1. Inmon WH. Tech topic: what is a data warehouse. [place unknown]: Prism Solutions;1995.2. Inmon WH, Derek S, Neushloss G. DW 2.0: the architecture for the next generation of data warehousing. Amsterdam: Morgan Kaufman;2008.3. Chaudhuri S, Dayal U. An overview of data warehousing and OLAP technology. ACM SIGMOD. 1997; 26(1):65–74.
Article4. Rubin DL, Desser TS. A data warehouse for integrating radiologic and pathologic data. J Am Coll Radiol. 2008; 5(3):210–217.
Article5. MacKenzie SL, Wyatt MC, Schuff R, Tenenbaum JD, Anderson N. Practices and perspectives on building integrated data repositories: results from a 2010 CTSA survey. J Am Med Inform Assoc. 2012; 19(e1):e119–e124.
Article6. Prather JC, Lobach DF, Goodwin LK, Hales JW, Hage ML, Hammond WE. Medical data mining: knowledge discovery in a clinical data warehouse. Proc AMIA Annu Symp. 1997; 1997:101–105.7. Ledbetter CS, Morgan MW. Toward best practice: leveraging the electronic patient record as a clinical data warehouse. J Healthc Inf Manag. 2001; 15(2):119–131.8. Dewitt JG, Hampton PM. Development of a data warehouse at an academic health system: knowing a place for the first time. Acad Med. 2005; 80(11):1019–1025.
Article9. Inmon B. Data warehousing in a healthcare environment. Pittsburgh (PA): The Data Administration Newsletter;2007. cited at 2014 Mar 20. Available from: http://www.tdan.com/view-articles/4584/.10. Evans RS, Lloyd JF, Pierce LA. Clinical use of an enterprise data warehouse. Proc AMIA Annu Symp. 2012; 2012:189–198.11. Embi PJ, Kaufman SE, Payne P. Biomedical informatics and outcomes research: enabling knowledge-driven health care. Circulation. 2009; 120(23):2393–2399.12. Jensen PB, Jensen LJ, Brunak S. Mining electronic health records: towards better research applications and clinical care. Nat Rev Genet. 2012; 13(6):395–405.
Article13. US Department of Health & Human Services. Guidance regarding methods for de-identification of protected health information in accordance with the Health Insurance Portability and Accountability Act (HIPAA) privacy rule. Washington (DC): US Department of Health & Human Service;c2013. cited at 2013 Apr 12. Available from: http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/De-identification/guidance.html.14. de Mul M, Alons P, van der Velde P, Konings I, Bakker J, Hazelzet J. Development of a clinical data warehouse from an intensive care clinical information system. Comput Methods Programs Biomed. 2012; 105(1):22–30.
Article15. Bernstam EV, Hersh WR, Johnson SB, Chute CG, Nguyen H, Sim I, et al. Synergies and distinctions between computational disciplines in biomedical research: perspective from the Clinical and Translational Science Award programs. Acad Med. 2009; 84(7):964–970.
Article16. Huser V, Cimino JJ. Desiderata for healthcare integrated data repositories based on architectural comparison of three public repositories. Proc AMIA Annu Symp. 2013; 2013:648–656.17. Liu J, Erdal S, Silvey SA, Ding J, Riedel JD, Marsh CB, et al. Toward a fully de-identified biomedical information warehouse. Proc AMIA Annu Symp. 2009; 2009:370–374.18. Kamal J, Liu J, Ostrander M, Santangelo J, Dyta R, Rogers P, et al. Information warehouse: a comprehensive informatics platform for business, clinical, and research applications. Proc AMIA Annu Symp. 2010; 2010:452–456.19. Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2). J Am Med Inform Assoc. 2010; 17(2):124–130.
Article20. Cincinnati Children's Hospital Medical Center. i2b2 Research data warehouse. Cincinnati (OH): Cincinnati Children's Hospital Medical Center. c2011. cited at 2014 Jan 8. Available from: https://i2b2.cchmc.org/.21. Lowe HJ, Ferris TA, Hernandez PM, Weber SC. STRIDE: an integrated standards-based translational research informatics platform. Proc AMIA Annu Symp. 2009; 2009:391–395.22. Hernandez P, Podchiyska T, Weber S, Ferris T, Lowe H. Automated mapping of pharmacy orders from two electronic health record systems to RxNorm within the STRIDE clinical data warehouse. Proc AMIA Annu Symp. 2009; 2009:244–248.23. Grant A, Moshyk A, Diab H, Caron P, de Lorenzi F, Bisson G, et al. Integrating feedback from a clinical data warehouse into practice organisation. Int J Med Inform. 2006; 75(3-4):232–239.
Article24. The University of Chicago, Center for Research Informatics. Clinical Research Data Warehouse (CRDW). Chicago (IL): The University of Chicago;c2014. cited at 2014 Mar 21. Available from: http://cri.uchicago.edu/?page_id=772/.25. Gray GW. Challenges of building clinical data analysis solutions. J Crit Care. 2004; 19(4):264–270.
Article26. Emory University. Clinical data warehouse - healthcare. Atlanta (GA): Emory University;c2014. cited at 2014 Mar 21. Available from: http://it.emory.edu/catalog/ehc_clinical_data_warehouse/.27. Kohane IS, Churchill SE, Murphy SN. A translational engine at the national scale: informatics for integrating biology and the bedside. J Am Med Inform Assoc. 2012; 19(2):181–185.
Article28. Kahn MG, Weng C. Clinical research informatics: a conceptual perspective. J Am Med Inform Assoc. 2012; 19(e1):e36–e42.
Article29. Dhir R, Patel AA, Winters S, Bisceglia M, Swanson D, Aamodt R, et al. A multidisciplinary approach to honest broker services for tissue banks and clinical data: a pragmatic and practical model. Cancer. 2008; 113(7):1705–1715.
Article30. El Emam K. Methods for the de-identification of electronic health records for genomic research. Genome Med. 2011; 3(4):25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Analysis of Clinical Information by Building the Clinical Data Warehouse
- Building a Lung and Ovarian Cancer Data Warehouse
- The Impact on the Musculoskeletal Symptoms of the Warehouse Employees's Work-related Characteristics and Job Stress
- The Strategic Planning of Hospital Management using Information Technology
- Analysis of Relationship between Levofloxacin and Corrected QT Prolongation Using a Clinical Data Warehouse