Healthc Inform Res.
2011 Mar;17(1):58-66. 10.4258/hir.2011.17.1.58.
Analysis of Relationship between Levofloxacin and Corrected QT Prolongation Using a Clinical Data Warehouse
- Affiliations
-
- 1Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea. veritas@ajou.ac.kr
- 2Department of Health Administration, School of Medical Information, Seokang University, Gwangju, Korea.
- 3Department of Information Technology, Gachon University of Medicine and Science, Incheon, Korea.
- 4Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Suwon, Korea.
- 5Department of Cardiology, Ajou University School of Medicine, Suwon, Korea.
- 6Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2166596
- DOI: http://doi.org/10.4258/hir.2011.17.1.58
Abstract
OBJECTIVE
The aim of this study was to examine whether or not levofloxacin has any relationship with QT prolongation in a real clinical setting by analyzing a clinical data warehouse of data collected from different hospital information systems.
METHODS
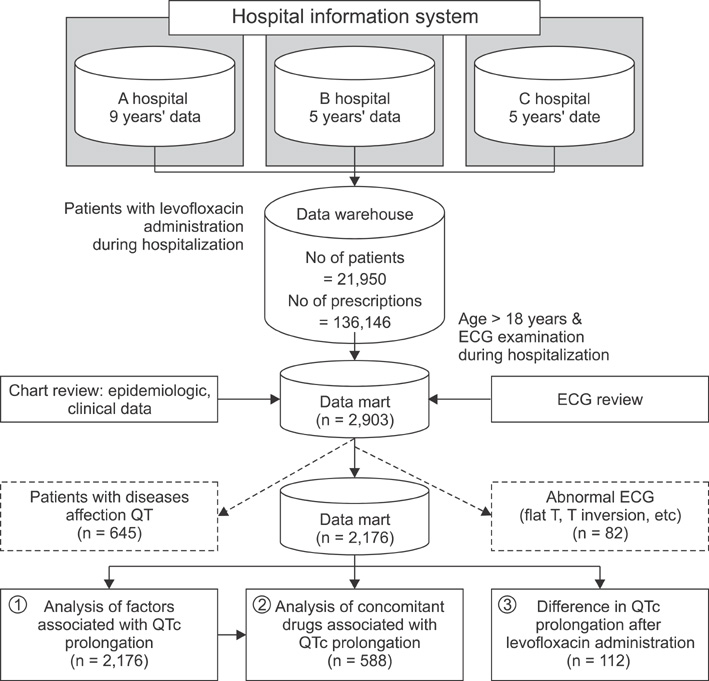
Electronic prescription data and medical charts from 3 different hospitals spanning the past 9 years were reviewed, and a clinical data warehouse was constructed. Patients who were both administrated levofloxacin and given electrocardiograms (ECG) were selected. The correlations between various patient characteristics, concomitant drugs, corrected QT (QTc) prolongation, and the interval difference in QTc before and after levofloxacin administration were analyzed.
RESULTS
A total of 2,176 patients from 3 different hospitals were included in the study. QTc prolongation was found in 364 patients (16.7%). The study revealed that age (OR 1.026, p < 0.001), gender (OR 0.676, p = 0.007), body temperature (OR 1.267, p = 0.024), and cigarette smoking (OR 1.641, p = 0.022) were related with QTc prolongation. After adjusting for related factors, 12 drugs concomitant with levofloxacin were associated with QTc prolongation. For patients who took ECGs before and after administration of levofloxacin during their hospitalization (n = 112), there was no significant difference in QTc prolongation.
CONCLUSIONS
The age, gender, body temperature, cigarette smoking and various concomitant drugs might be related with QTc prolongation. However, there was no definite causal relationship or interaction between levofloxacin and QTc prolongation. Alternative surveillance methods utilizing the massive accumulation of electronic medical data seem to be essential to adverse drug reaction surveillance in future.
Keyword
MeSH Terms
Figure
Reference
-
1. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003. 89:1363–1372.
Article2. Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A Jr. The long QT syndrome: prospective longitudinal study of 328 families. Circulation. 1991. 84:1136–1144.
Article3. Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2003. 2:439–447.
Article4. Woosley RL. Cardiac actions of antihistamines. Annu Rev Pharmacol Toxicol. 1996. 36:233–252.
Article5. Yap YG, Camm AJ. The current cardiac safety situation with antihistamines. Clin Exp Allergy. 1999. 29:Suppl 1. 15–24.
Article6. QT drug lists by risk groups. Arizona Center for Education and Research on Therapeutics. cited 2011 Mar 29. Arizona Center for Education and Research on Therapeutics;Available from: http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm.7. Brown AM. Drugs, hERG and sudden death. Cell Calcium. 2004. 35:543–547.
Article8. Ahmad SR. Adverse drug event monitoring at the food and drug administration. J Gen Intern Med. 2003. 18:57–60.
Article9. Waller PC, Evans SJ. A model for the future conduct of pharmacovigilance. Pharmacoepidemiol Drug Saf. 2003. 12:17–29.
Article10. Weaver J, Bonnel RA, Karwoski CB, Brinker AD, Beitz J. GI events leading to death in association with celecoxib and rofecoxib. Am J Gastroenterol. 2001. 96:3449–3450.
Article11. DesRoches CM, Campbell EG, Rao SR, Donelan K, Ferris TG, Jha A, Kaushal R, Levy DE, Rosenbaum S, Shields AE, Blumenthal D. Electronic health records in ambulatory care: a national survey of physicians. N Engl J Med. 2008. 359:50–60.
Article12. Park RW, Shin SS, Choi Yl, Ahn JO, Hwang SC. Computerized physician order entry and electronic medical record systems in Korean teaching and general hospitals: results of a 2004 survey. J Am Med Inform Assoc. 2005. 12:642–647.
Article13. Hauben M, Patadia V, Gerrits C, Walsh L, Reich L. Data mining in pharmacovigilance: the need for a balanced perspective. Drug Saf. 2005. 28:835–842.14. Dewitt JG, Hampton PM. Development of a data warehouse at an academic health system: knowing a place for the first time. Acad Med. 2005. 80:1019–1025.
Article15. Zhang Q, Matsumura Y, Teratani T, Yoshimoto S, Mineno T, Nakagawa K, Nagahama M, Kuwata S, Takeda H. The application of an institutional clinical data warehouse to the assessment of adverse drug reactions (ADRs): evaluation of aminoglycoside and cephalosporin associated nephrotoxicity. Methods Inf Med. 2007. 46:516–522.
Article16. Szirbik NB, Pelletier C, Chaussalet T. Six methodological steps to build medical data warehouses for research. Int J Med Inform. 2006. 75:683–691.
Article17. Sheen SS, Choi JE, Park RW, Kim EY, Lee YH, Kang UG. Overdose rate of drugs requiring renal dose adjustment: data analysis of 4 years prescriptions at a tertiary teaching hospital. J Gen Intern Med. 2008. 23:423–428.
Article18. Hinrichsen VL, Kruskal B, O'Brien MA, Lieu TA, Platt R. Using electronic medical records to enhance detection and reporting of vaccine adverse events. J Am Med Inform Assoc. 2007. 14:731–735.
Article19. Akita M, Shibazaki Y, Izumi M, Hiratsuka K, Sakai T, Kurosawa T, Shindo Y. Comparative assessment of prurifloxacin, sparfloxacin, gatifloxacin and levofloxacin in the rabbit model of proarrhythmia. J Toxicol Sci. 2004. 29:63–71.
Article20. Nykamp DL, Blackmon CL, Schmidt PE, Roberson AG. QTc prolongation associated with combination therapy of levofloxacin, imipramine, and fluoxetine. Ann Pharmacother. 2005. 39:543–546.
Article21. Gandhi PJ, Menezes PA, Vu HT, Rivera AL, Ramaswamy K. Fluconazole- and levofloxacin-induced torsades de pointes in an intensive care unit patient. Am J Health Syst Pharm. 2003. 60:2479–2483.
Article22. Taubel J, Naseem A, Harada T, Wang D, Arezina R, Lorch U, Camm AJ. Levofloxacin can be used effectively as a positive control in thorough QT/QTc studies in healthy volunteers. Br J Clin Pharmacol. 2010. 69:391–400.
Article23. Tsikouris JP, Peeters MJ, Cox CD, Meyerrose GE, Seifert CF. Effects of three fluoroquinolones on QT analysis after standard treatment courses. Ann Noninvasive Electrocardiol. 2006. 11:52–56.
Article24. Park MY, Lee YH, Kim EY, Kim WJ, Kam HJ, Choi JP, Han TH, Kang UG, Park RW. A data warehouse based retrospective post-marketing surveillance method: a feasibility test with fluoxetine. J Korean Soc Med Inform. 2009. 15:191–199.
Article25. Bazett HC. The time relations of the blood-pressure changes after excision of the adrenal glands, with some observations on blood volume changes. J Physiol. 1920. 53:320–339.
Article26. Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999. 354:1625–1633.
Article27. Mattu A, Brady WJ, Perron AD. Electrocardiographic manifestations of hypothermia. Am J Emerg Med. 2002. 20:314–326.
Article28. Singh K. Effect of smoking on QT interval, QT dispersion and rate pressure product. Indian Heart J. 2004. 56:140–142.29. Suzuki M, Nishizaki M, Arita M, Ashikaga T, Yamawake N, Kakuta T, Numano F, Hiraoka M. Increased QT dispersion in patients with vasospastic angina. Circulation. 1998. 98:435–440.
Article30. Radhakrishnan M, Agarwal S, Bithal PK, Gupta V. Heparin-induced transient prolongation of the QT interval during endovascular embolisation of intracranial aneurysm. J Clin Neurosci. 2006. 13:489–492.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of a Risk Score for QT Prolongation in the Intensive Care Unit Using Time-Series Electrocardiogram Data and Electronic Medical Records
- A Data Warehouse Based Retrospective Post-marketing Surveillance Method: A Feasibility Test with Fluoxetine
- QT-interval prolongation due to medication found in the preoperative evaluation
- A Case of Giant T wave and QT Prolongation Associated with Acute Pulmonary Edema
- QTc prolongation in patients with COVID-19: a retrospective chart review